Methadone but not morphine inhibits lubiprostone-stimulated Cl- currents in T84 intestinal cells and recombinant human ClC-2, but not CFTR Cl- currents
- PMID: 22918821
- PMCID: PMC3627040
- DOI: 10.1007/s12013-012-9406-6
Methadone but not morphine inhibits lubiprostone-stimulated Cl- currents in T84 intestinal cells and recombinant human ClC-2, but not CFTR Cl- currents
Abstract
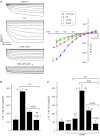
In clinical trials, methadone, but not morphine, appeared to prevent beneficial effects of lubiprostone, a ClC-2 Cl(-) channel activator, on opioid-induced constipation. Effects of methadone and morphine on lubiprostone-stimulated Cl(-) currents were measured by short circuit current (Isc) across T84 cells. Whole cell patch clamp of human ClC-2 (hClC-2) stably expressed in HEK293 cells and in a high expression cell line (HEK293EBNA) as well as human CFTR (hCFTR) stably expressed in HEK293 cells was used to study methadone and morphine effects on recombinant hClC-2 and hCFTR Cl(-) currents. Methadone but not morphine inhibited lubiprostone-stimulated Isc in T84 cells with half-maximal inhibition at 100 nM. Naloxone did not affect lubiprostone stimulation or methadone inhibition of Isc. Lubiprostone-stimulated Cl(-) currents in hClC-2/HEK293 cells, but not forskolin/IBMX-stimulated Cl(-) currents in hCFTR/HEK293 cells, were inhibited by methadone, but not morphine. HEK293EBNA cells expressing hClC-2 showed time-dependent, voltage-activated, CdCl2-inhibited Cl(-) currents in the absence (control) and the presence of lubiprostone. Methadone, but not morphine, inhibited control and lubiprostone-stimulated hClC-2 Cl(-) currents with half-maximal inhibition at 100 and 200-230 nM, respectively. Forskolin/IBMX-stimulated hClC-2 Cl(-) currents were also inhibited by methadone. Myristoylated protein kinase inhibitor (a specific PKA inhibitor) inhibited forskolin/IBMX- but not lubiprostone-stimulated hClC-2 Cl(-) currents. Methadone caused greater inhibition of lubiprostone-stimulated currents added before patching (66.1 %) compared with after patching (28.7 %). Methadone caused inhibition of lubiprostone-stimulated Cl(-) currents in T84 cells and control; lubiprostone- and forskolin/IBMX-stimulated recombinant hClC-2 Cl(-) currents may be the basis for reduced efficacy of lubiprostone in methadone-treated patients.
Figures





References
-
- Cryer, B., Katz, S., Vallejo, C., Scott, C., Joswick, TR., Dolecek, G. (2010). A phase 3, randomized, double-blind, placebo-controlled clinical trial of lubiprostone for the treatment of opioid-induced bowel dysfunction in patients with chronic, non-cancer pain. Gastroenterology, 138, S-129 [abst 906].
-
- Jamal MM, Mareya SM, Woldegeorgis F, Joswick TR, Ueno R. Lubiprostone significantly improves treatment response in non-methadone opioid-induced bowel dysfunction patients with chronic, non-cancer pain: results from a Phase 3, randomized, double-blind, placebo-controlled clinical trial. Gastroenterology. 2012;142:S144–S145.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

