How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma
- PMID: 22918923
- PMCID: PMC6278954
- DOI: 10.1093/annonc/mds258
How recent advances in immunotherapy are changing the standard of care for patients with metastatic melanoma
Abstract
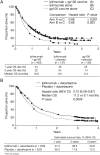
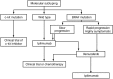
In 2011, two therapies were approved for the treatment of metastatic melanoma: ipilimumab, an immunotherapeutic agent, and vemurafenib, a BRAF kinase inhibitor. These approvals were based on data from phase III trials, which showed that treatment with these agents produced substantial improvements in overall survival (OS). Ipilimumab has been investigated in two phase III trials: one as monotherapy in patients with pretreated metastatic melanoma at a dose of 3 mg/kg and the second in combination with dacarbazine (DTIC) chemotherapy in patients with previously untreated metastatic melanoma at a dose of 10 mg/kg. Among the pretreated patients, ipilimumab monotherapy significantly improved median OS (Hazard ratio (HR): 0.66, P = 0.003) from 6.4 months in gp100 vaccine controls to 10.1 months. The rates of OS in the ipilimumab-alone group and the gp100 group, respectively, were 45.6% and 25.3% at 12 months and 23.5% and 13.7% at 24 months. In the second trial, OS was significantly longer in previously untreated patients receiving ipilimumab plus DTIC than those receiving DTIC plus placebo (11.2 months versus 9.1 months; HR: 0.72, P < 0.001), with higher survival rates in the ipilimumab plus DTIC group at 1 year (47.3% versus 36.3%), 2 years (28.5% versus 17.9%) and 3 years (20.8% versus 12.2%). When using ipilimumab in the clinic, special consideration should be given to immune-related adverse events (irAEs) and assessment of response. Established guidelines can be used to manage the majority of irAEs effectively. Proposed modifications made to the existing response criteria mean that the clinician can accurately detect immune-related responses that would have been considered representative of progressive disease using conventional criteria. Further research is warranted to establish how immunotherapeutic agents can be combined with conventional agents, with each other or with molecularly targeted agents such as vemurafenib, to further optimise clinical outcomes.
Figures

References
-
- Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313:1485–1492. - PubMed
-
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

