APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing
- PMID: 22918942
- PMCID: PMC3469513
- DOI: 10.1091/mbc.E12-05-0352
APC(FZR1) prevents nondisjunction in mouse oocytes by controlling meiotic spindle assembly timing
Abstract
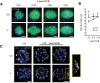
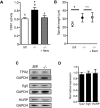
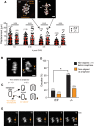
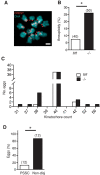
FZR1 is an anaphase-promoting complex (APC) activator best known for its role in the mitotic cell cycle at M-phase exit, in G1, and in maintaining genome integrity. Previous studies also established that it prevents meiotic resumption, equivalent to the G2/M transition. Here we report that mouse oocytes lacking FZR1 undergo passage through meiosis I that is accelerated by ~1 h, and this is due to an earlier onset of spindle assembly checkpoint (SAC) satisfaction and APC(CDC20) activity. However, loss of FZR1 did not compromise SAC functionality; instead, earlier SAC satisfaction was achieved because the bipolar meiotic spindle was assembled more quickly in the absence of FZR1. This novel regulation of spindle assembly by FZR1 led to premature bivalent attachment to microtubules and loss of kinetochore-bound MAD2. Bivalents, however, were observed to congress poorly, leading to nondisjunction rates of 25%. We conclude that in mouse oocytes FZR1 controls the timing of assembly of the bipolar spindle and in so doing the timing of SAC satisfaction and APC(CDC20) activity. This study implicates FZR1 as a major regulator of prometaphase whose activity helps to prevent chromosome nondisjunction.
Figures

Similar articles
-
BubR1 is a spindle assembly checkpoint protein regulating meiotic cell cycle progression of mouse oocyte.Cell Cycle. 2010 Mar 15;9(6):1112-21. doi: 10.4161/cc.9.6.10957. Epub 2010 Mar 15. Cell Cycle. 2010. PMID: 20237433
-
Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase.J Cell Biol. 1999 Jul 12;146(1):1-12. doi: 10.1083/jcb.146.1.1. J Cell Biol. 1999. PMID: 10402455 Free PMC article.
-
Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension.Development. 2012 Jun;139(11):1947-55. doi: 10.1242/dev.077040. Epub 2012 Apr 18. Development. 2012. PMID: 22513370
-
Spindle formation, chromosome segregation and the spindle checkpoint in mammalian oocytes and susceptibility to meiotic error.Mutat Res. 2008 Mar 12;651(1-2):14-29. doi: 10.1016/j.mrgentox.2007.10.015. Epub 2007 Nov 9. Mutat Res. 2008. PMID: 18096427 Review.
-
Dual inhibition of Cdc20 by the spindle checkpoint.J Biomed Sci. 2007 Jul;14(4):475-9. doi: 10.1007/s11373-007-9157-3. Epub 2007 Mar 17. J Biomed Sci. 2007. PMID: 17370142 Review.
Cited by
-
Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I.J Cell Biol. 2013 Jul 22;202(2):221-9. doi: 10.1083/jcb.201303019. Epub 2013 Jul 15. J Cell Biol. 2013. PMID: 23857768 Free PMC article.
-
The spindle checkpoint and chromosome segregation in meiosis.FEBS J. 2015 Jul;282(13):2471-87. doi: 10.1111/febs.13166. Epub 2015 Jan 12. FEBS J. 2015. PMID: 25470754 Free PMC article. Review.
-
The role of Anaphase Promoting Complex activation, inhibition and substrates in cancer development and progression.Aging (Albany NY). 2020 Aug 15;12(15):15818-15855. doi: 10.18632/aging.103792. Epub 2020 Aug 15. Aging (Albany NY). 2020. PMID: 32805721 Free PMC article. Review.
-
The small non-coding RNA profile of mouse oocytes is modified during aging.Aging (Albany NY). 2019 May 24;11(10):2968-2997. doi: 10.18632/aging.101947. Aging (Albany NY). 2019. PMID: 31128574 Free PMC article.
-
Chronic restraint stress disturbs meiotic resumption through APC/C-mediated cyclin B1 excessive degradation in mouse oocytes.Cell Cycle. 2018;17(13):1591-1601. doi: 10.1080/15384101.2018.1471316. Epub 2018 Aug 4. Cell Cycle. 2018. PMID: 29911914 Free PMC article.
References
-
- Brunet S, Pahlavan G, Taylor S, Maro B. Functionality of the spindle checkpoint during the first meiotic division of mammalian oocytes. Reproduction. 2003;126:443–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

