Role of mitochondrial inner membrane organizing system in protein biogenesis of the mitochondrial outer membrane
- PMID: 22918945
- PMCID: PMC3469511
- DOI: 10.1091/mbc.E12-04-0295
Role of mitochondrial inner membrane organizing system in protein biogenesis of the mitochondrial outer membrane
Abstract
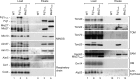
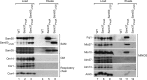
Mitochondria contain two membranes, the outer membrane and the inner membrane with folded cristae. The mitochondrial inner membrane organizing system (MINOS) is a large protein complex required for maintaining inner membrane architecture. MINOS interacts with both preprotein transport machineries of the outer membrane, the translocase of the outer membrane (TOM) and the sorting and assembly machinery (SAM). It is unknown, however, whether MINOS plays a role in the biogenesis of outer membrane proteins. We have dissected the interaction of MINOS with TOM and SAM and report that MINOS binds to both translocases independently. MINOS binds to the SAM complex via the conserved polypeptide transport-associated domain of Sam50. Mitochondria lacking mitofilin, the large core subunit of MINOS, are impaired in the biogenesis of β-barrel proteins of the outer membrane, whereas mutant mitochondria lacking any of the other five MINOS subunits import β-barrel proteins in a manner similar to wild-type mitochondria. We show that mitofilin is required at an early stage of β-barrel biogenesis that includes the initial translocation through the TOM complex. We conclude that MINOS interacts with TOM and SAM independently and that the core subunit mitofilin is involved in biogenesis of outer membrane β-barrel proteins.
Figures




Similar articles
-
Role of MINOS in mitochondrial membrane architecture: cristae morphology and outer membrane interactions differentially depend on mitofilin domains.J Mol Biol. 2012 Sep 14;422(2):183-91. doi: 10.1016/j.jmb.2012.05.004. Epub 2012 May 7. J Mol Biol. 2012. PMID: 22575891
-
Sam37 is crucial for formation of the mitochondrial TOM-SAM supercomplex, thereby promoting β-barrel biogenesis.J Cell Biol. 2015 Sep 28;210(7):1047-54. doi: 10.1083/jcb.201504119. J Cell Biol. 2015. PMID: 26416958 Free PMC article.
-
Alternative function for the mitochondrial SAM complex in biogenesis of alpha-helical TOM proteins.J Cell Biol. 2007 Dec 3;179(5):881-93. doi: 10.1083/jcb.200706043. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039934 Free PMC article.
-
Biogenesis of mitochondrial β-barrel membrane proteins.FEBS Open Bio. 2024 Oct;14(10):1595-1609. doi: 10.1002/2211-5463.13905. Epub 2024 Sep 29. FEBS Open Bio. 2024. PMID: 39343721 Free PMC article. Review.
-
Coupling of import and assembly pathways in mitochondrial protein biogenesis.Biol Chem. 2019 Dec 18;401(1):117-129. doi: 10.1515/hsz-2019-0310. Biol Chem. 2019. PMID: 31513529 Review.
Cited by
-
Sub-mitochondrial localization of the genetic-tagged mitochondrial intermembrane space-bridging components Mic19, Mic60 and Sam50.J Cell Sci. 2017 Oct 1;130(19):3248-3260. doi: 10.1242/jcs.201400. Epub 2017 Aug 14. J Cell Sci. 2017. PMID: 28808085 Free PMC article.
-
Mitochondrial proteins: from biogenesis to functional networks.Nat Rev Mol Cell Biol. 2019 May;20(5):267-284. doi: 10.1038/s41580-018-0092-0. Nat Rev Mol Cell Biol. 2019. PMID: 30626975 Free PMC article. Review.
-
Structures and functions of the MICOS: Pathogenesis and therapeutic implications in Alzheimer's disease.Acta Pharm Sin B. 2025 Jun;15(6):2966-2984. doi: 10.1016/j.apsb.2025.04.019. Epub 2025 Apr 22. Acta Pharm Sin B. 2025. PMID: 40654371 Free PMC article. Review.
-
Ghost mitochondria drive metastasis through adaptive GCN2/Akt therapeutic vulnerability.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2115624119. doi: 10.1073/pnas.2115624119. Proc Natl Acad Sci U S A. 2022. PMID: 35177476 Free PMC article.
-
Cox17 Protein Is an Auxiliary Factor Involved in the Control of the Mitochondrial Contact Site and Cristae Organizing System.J Biol Chem. 2015 Jun 12;290(24):15304-12. doi: 10.1074/jbc.M115.645069. Epub 2015 Apr 27. J Biol Chem. 2015. PMID: 25918166 Free PMC article.
References
-
- Banci L, Bertini I, Cefaro C, Ciofi-Baffoni S, Gallo A, Martinelli M, Sideris DP, Katrakili N, Tokatlidis K. MIA40 is an oxidoreductase that catalyzes oxidative protein folding in mitochondria. Nat Struct Mol Biol. 2009;16:198–206. - PubMed
-
- Becker T, Böttinger L, Pfanner N. Mitochondrial protein import: from transport pathways to an integrated network. Trends Biochem Sci. 2012;37:85–91. - PubMed
-
- Becker T, Gebert M, Pfanner N, van der Laan M. Biogenesis of mitochondrial membrane proteins. Curr Opin Cell Biol. 2009;21:484–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

