Differential wiring of layer 2/3 neurons drives sparse and reliable firing during neocortical development
- PMID: 22918982
- PMCID: PMC3792743
- DOI: 10.1093/cercor/bhs257
Differential wiring of layer 2/3 neurons drives sparse and reliable firing during neocortical development
Abstract
Sensory information is transmitted with high fidelity across multiple synapses until it reaches the neocortex. There, individual neurons exhibit enormous variability in responses. The source of this diversity in output has been debated. Using transgenic mice expressing the green fluorescent protein coupled to the activity-dependent gene c-fos, we identified neurons with a history of elevated activity in vivo. Focusing on layer 4 to layer 2/3 connections, a site of strong excitatory drive at an initial stage of cortical processing, we find that fluorescently tagged neurons receive significantly greater excitatory and reduced inhibitory input compared with neighboring, unlabeled cells. Differential wiring of layer 2/3 neurons arises early in development and requires sensory input to be established. Stronger connection strength is not associated with evidence for recent synaptic plasticity, suggesting that these more active ensembles may not be generated over short time scales. Paired recordings show fosGFP+ neurons spike at lower stimulus thresholds than neighboring, fosGFP- neurons. These data indicate that differences in circuit construction can underlie response heterogeneity amongst neocortical neurons.
Keywords: AMPA receptors; activity-dependent gene expression; critical period; somatosensory; subnetworks.
Figures




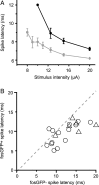

References
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science (New York, NY) 1996;273:1868–1871. - PubMed
-
- Aronoff R, Matyas F, Mateo C, Ciron C, Schneider B, Petersen CC. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. - PubMed
-
- Barth AL, Poulet JF. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 2012;35:345–355. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

