Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages
- PMID: 22919031
- PMCID: PMC3471522
- DOI: 10.1182/blood-2012-04-422758
Endothelial cells provide an instructive niche for the differentiation and functional polarization of M2-like macrophages
Abstract
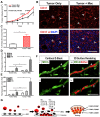
Endothelial cells and macrophages are known to engage in tight and specific interactions that contribute to the modulation of vascular function. Here we show that adult endothelial cells provide critical signals for the selective growth and differentiation of macrophages from several hematopoietic progenitors. The process features the formation of well-organized colonies that exhibit progressive differentiation from the center to the periphery and toward an M2-like phenotype, characterized by enhanced expression of Tie2 and CD206/Mrc1. These colonies are long-lived depending on the contact with the endothelium; removal of the endothelial monolayer results in rapid colony dissolution. We further found that Csf1 produced by the endothelium is critical for the expansion of the macrophage colonies and that blockade of Csf1 receptor impairs colony growth. Functional analyses indicate that these macrophages are capable of accelerating angiogenesis, promoting tumor growth, and effectively engaging in tight associations with endothelial cells in vivo. These findings uncover a critical role of endothelial cells in the induction of macrophage differentiation and their ability to promote further polarization toward a proangiogenic phenotype. This work also highlights some of the molecules underlying the M2-like differentiation, a process that is relevant to the progression of both developmental and pathologic angiogenesis.
Figures

References
-
- Eilken H, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457(7231):896–900. - PubMed
-
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

