Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization
- PMID: 22919059
- PMCID: PMC3473205
- DOI: 10.1210/en.2012-1458
Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization
Abstract
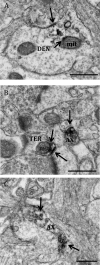
Estrogens rapidly affect dopamine (DA) neurotransmission in the dorsal striatum (dSTR) and DA-related diseases, such as Parkinson's disease and schizophrenia. How estrogens influence DA function remains unclear, in part, because the ultrastructural localization of estrogen receptors (ER) in the dSTR is not known. Light microscopic studies of the dSTR have suggested the presence of ER. This experiment used electron microscopy to determine whether these ER are at extranuclear sites in the dSTR, providing evidence for a mechanism through which estrogen could rapidly affect DA transmission. The dSTR was labeled with antibodies for ERα, ERβ, and G protein-coupled ER 1 (GPER-1) to confirm whether these ER were present in this brain area. After this, the dSTR was dual labeled with antibodies for ERα or GPER-1 and tyrosine hydroxylase or vesicular acetylcholine transporter to determine whether ER are localized to dopaminergic and/or cholinergic processes, respectively. Ultrastructural analysis revealed immunoreactivity (IR) for ERα, ERβ, and GPER-1 exclusively at extranuclear sites throughout the dSTR. ERα-, ERβ-, and GPER-1-IR are mostly frequently observed in axons and glial profiles but are also localized to other neuronal profiles. Dual labeling revealed that ERα- and GPER-1-IR is not associated with DA axons and terminals but is sometimes associated with cholinergic neurons. Because these receptors are exclusively extranuclear in the dSTR, binding at these receptors likely affects neurotransmission via nongenomic mechanisms.
Figures


References
-
- Quinlan MG, Hussain D, Brake WG. 2008. Use of cognitive strategies in rats: the role of estradiol and its interaction with dopamine. Horm Behav 53:185–191 - PubMed
-
- Nofrey BS, Ben-Shahar OM, Brake WG. 2008. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn 66:156–160 - PubMed
-
- Quinlan MG, Duncan A, Loiselle C, Graffe N, Brake WG. 2010. Latent inhibition is affected by phase of estrous cycle in female rats. Brain Cogn 74:244–248 - PubMed
-
- Bourque M, Dluzen DE, Di Paolo T. 2009. Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol 30:142–157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

