Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers
- PMID: 22919122
- PMCID: PMC3423226
- DOI: 10.1021/cm2028367
Review of Long-Wavelength Optical and NIR Imaging Materials: Contrast Agents, Fluorophores and Multifunctional Nano Carriers
Abstract
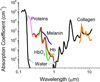
The importance of long wavelength and near infra-red (NIR) imaging has dramatically increased due to the desire to perform whole animal and deep tissue imaging. The adoption of NIR imaging is also growing rapidly due to the availability of targeted biological agents for diagnosis and basic medical research that can be imaged in vivo. The wavelength range of 650-1450 nm falls in the region of the spectrum with the lowest absorption in tissue and therefore enables the deepest tissue penetration. This is the wavelength range we focus on with this review. To operate effectively the imaging agents must both be excited and must emit in this long-wavelength window. We review the agents used both for imaging by absorption, scattering, and excitation (such as fluorescence). Imaging agents comprise both aqueous soluble and insoluble species, both organic and inorganic, and unimolecular and supramolecular constructs. The interest in multi-modal imaging, which involves delivery of actives, targeting, and imaging, requires nanocarriers or supramolecular assemblies. Nanoparticles for diagnostics also have advantages in increasing circulation time and increased imaging brightness relative to single molecule imaging agents. This has led to rapid advances in nanocarriers for long-wavelength, NIR imaging.
Figures


 3 µm |
3 µm |  9 µm |
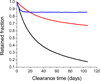
9 µm |  15 µm). Radioactively tagged polystyrene particles, intratracheally administered, were monitored for clearance from the lung. Adapted from Oberdörster (1989) and Snipes (1981).,
15 µm). Radioactively tagged polystyrene particles, intratracheally administered, were monitored for clearance from the lung. Adapted from Oberdörster (1989) and Snipes (1981)., 








References
-
- Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, Altman H, Keidar Z, Israel O. Clinical Performance of PET/CT in Evaluation of Cancer: Additional Value for Diagnostic Imaging and Patient Management. Journal of Nuclear Medicine. 2003;44(8):1200–1209. - PubMed
-
- Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, Jerin J, Young J, Byars L, Nutt R. A Combined PET/CT Scanner for Clinical Oncology. Journal of Nuclear Medicine. 2000;41(8):1369–1379. - PubMed
-
- DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance Characteristics of a Whole-Body PET Scanner. Journal of Nuclear Medicine. 1994;35(8):1398–1406. - PubMed
-
- Strauss LG, Conti PS. The Applications of PET in Clinical Oncology. Journal of Nuclear Medicine. 1991;32(4):623–648. - PubMed
-
- Pichler BJ, Kolb A, Nagele T, Schlemmer HP. PET/MRI: Paving the Way for the Next Generation of Clinical Multimodality Imaging Applications. Journal of Nuclear Medicine. 2010;51(3):333–336. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
