Assessment of bio-safety of low-cost polyurethane urologic stents used in developing countries
- PMID: 22919135
- PMCID: PMC3424896
- DOI: 10.4103/0970-1591.98462
Assessment of bio-safety of low-cost polyurethane urologic stents used in developing countries
Abstract
Background: Ureteral stents, despite their ubiquitous use, have not been evaluated for their safety and strength after removal from the patient. While literature is available from the industry with regards to manufacturing and specifications of stents, what happens to a stent after it is inserted into the body, still needs to be explored.
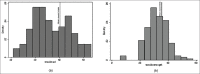
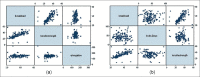
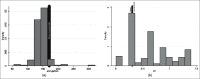
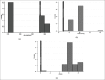
Materials and methods: We conducted a methodical study of 153 consecutive patients with urological problems who were stented with inexpensive polyurethane stents. Once removed from the patients, the stents were analyzed for breakload, tensile strength, elongation, pH, decomposition temperature, residue as well as diameter change.
Results: There was no significant change in the physical and mechanical properties of the stent after clinical use and the variance was within the acceptable range of biomaterials. There was minimal leaching of material and color change in all stents.
Conclusion: The cheap polyurethane stents were found to be safe for use in patients, for the short time periods of in situ stenting. The degradation of physical and chemical properties of the stent was not significant. Thus it can be safely said that the stents currently in widespread use are cost-effective and physically safe for short spans of time.
Keywords: Cost-effective stents; polyurethane DJ stents; ureteral stents.
Conflict of interest statement
Figures
Similar articles
-
Mechanical performance of polyurethane ureteral stents in vitro and ex vivo.Biomaterials. 1997 Oct;18(20):1379-83. doi: 10.1016/s0142-9612(97)00070-7. Biomaterials. 1997. PMID: 9363338
-
Applications and complications of polyurethane stenting in urology.J Ayub Med Coll Abbottabad. 2006 Apr-Jun;18(2):69-72. J Ayub Med Coll Abbottabad. 2006. PMID: 16977819
-
Outcomes and costs analysis of Externalized PyeloUreteral versus internal Double-J ureteral stents after paediatric laparoscopic Anderson-Hynes pyeloplasty.J Pediatr Urol. 2021 Apr;17(2):232.e1-232.e7. doi: 10.1016/j.jpurol.2020.12.006. Epub 2020 Dec 8. J Pediatr Urol. 2021. PMID: 33388262
-
Comparative evaluation of materials used for internal ureteral stents.J Endourol. 1993 Apr;7(2):105-15. doi: 10.1089/end.1993.7.105. J Endourol. 1993. PMID: 8518822 Review.
-
Prospects for the research and application of biodegradable ureteral stents: from bench to bedside.J Biomater Sci Polym Ed. 2018 Oct;29(14):1657-1666. doi: 10.1080/09205063.2018.1498184. Epub 2018 Sep 25. J Biomater Sci Polym Ed. 2018. PMID: 30141744 Review.
References
-
- Finney RP. Experience with new double J ureteral catheter stent. J Urol. 1978;120:678–81. - PubMed
-
- Beiko DT, Knudsen BE, Denstedt JD. Advances in ureteral stent design. J Endourol. 2003;17:195–9. - PubMed
-
- Van Arsdalen KN, Pollack HM, Wein AJ. Ureteral Stenting. Semin Urol. 1984;2:180–6. - PubMed
-
- Chen AS, Saltzman B. Stent use with extracorporeal shock wave lithotripsy. J Endourol. 1993;7:155–62. - PubMed
-
- Denstedt JD, Reid G, Sofer M. Advances in ureteral stent technology. World J Urol. 2000;18:237–42. - PubMed

