Structure and function of the Haemophilus influenzae autotransporters
- PMID: 22919571
- PMCID: PMC3417375
- DOI: 10.3389/fcimb.2011.00005
Structure and function of the Haemophilus influenzae autotransporters
Abstract
Autotransporters are a large class of proteins that are found in the outer membrane of Gram-negative bacteria and are almost universally implicated in virulence. These proteins consist of a C-terminal β-domain that is embedded in the outer membrane and an N-terminal domain that is exposed on the bacterial surface and is endowed with effector function. In this article, we review and compare the structural and functional characteristics of the Haemophilus influenzae IgA1 protease and Hap monomeric autotransporters and the H. influenzae Hia and Hsf trimeric autotransporters. All of these proteins play a role in colonization of the upper respiratory tract and in the pathogenesis of H. influenzae disease.
Keywords: Haemophilus influenzae; adhesin; autotransporter; serine protease.
Figures


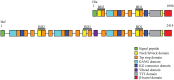

Similar articles
-
Repetitive architecture of the Haemophilus influenzae Hia trimeric autotransporter.J Mol Biol. 2008 Dec 26;384(4):824-36. doi: 10.1016/j.jmb.2008.09.085. Epub 2008 Oct 11. J Mol Biol. 2008. PMID: 18948113 Free PMC article.
-
Protein folding in bacterial adhesion: secretion and folding of classical monomeric autotransporters.Adv Exp Med Biol. 2011;715:125-42. doi: 10.1007/978-94-007-0940-9_8. Adv Exp Med Biol. 2011. PMID: 21557061 Review.
-
Structure of the outer membrane translocator domain of the Haemophilus influenzae Hia trimeric autotransporter.EMBO J. 2006 Jun 7;25(11):2297-304. doi: 10.1038/sj.emboj.7601132. Epub 2006 May 11. EMBO J. 2006. PMID: 16688217 Free PMC article.
-
The Haemophilus influenzae Hap autotransporter binds to fibronectin, laminin, and collagen IV.Infect Immun. 2002 Sep;70(9):4902-7. doi: 10.1128/IAI.70.9.4902-4907.2002. Infect Immun. 2002. PMID: 12183535 Free PMC article.
-
The pathogenesis of nontypable Haemophilus influenzae otitis media.Vaccine. 2000 Dec 8;19 Suppl 1:S41-50. doi: 10.1016/s0264-410x(00)00277-2. Vaccine. 2000. PMID: 11163462 Review.
Cited by
-
The Role of Bacterial Secretion Systems in the Virulence of Gram-Negative Airway Pathogens Associated with Cystic Fibrosis.Front Microbiol. 2016 Aug 30;7:1336. doi: 10.3389/fmicb.2016.01336. eCollection 2016. Front Microbiol. 2016. PMID: 27625638 Free PMC article. Review.
-
Neisseria gonorrhoeae NGO2105 Is an Autotransporter Protein Involved in Adhesion to Human Cervical Epithelial Cells and in vivo Colonization.Front Microbiol. 2020 Jun 25;11:1395. doi: 10.3389/fmicb.2020.01395. eCollection 2020. Front Microbiol. 2020. PMID: 32670242 Free PMC article.
-
Utilization of Variant and Fusion Proteins To Functionally Map the Aggregatibacter actinomycetemcomitans Trimeric Autotransporter Protein ApiA.Infect Immun. 2018 Feb 20;86(3):e00697-17. doi: 10.1128/IAI.00697-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29229732 Free PMC article.
-
Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains.Clin Vaccine Immunol. 2014 May;21(5):613-21. doi: 10.1128/CVI.00772-13. Epub 2014 Feb 26. Clin Vaccine Immunol. 2014. PMID: 24574538 Free PMC article.
-
Relative Contribution of P5 and Hap Surface Proteins to Nontypable Haemophilus influenzae Interplay with the Host Upper and Lower Airways.PLoS One. 2015 Apr 20;10(4):e0123154. doi: 10.1371/journal.pone.0123154. eCollection 2015. PLoS One. 2015. PMID: 25894755 Free PMC article.
References
-
- Bachovchin W. W., Plaut A. G., Flentke G. R., Lynch M., Kettner C. A. (1990). Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J. Biol. Chem. 265, 3738–3743 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

