Brucella ceti and brucellosis in cetaceans
- PMID: 22919595
- PMCID: PMC3417395
- DOI: 10.3389/fcimb.2012.00003
Brucella ceti and brucellosis in cetaceans
Abstract
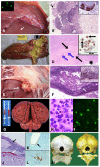
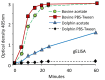
Since the first case of brucellosis detected in a dolphin aborted fetus, an increasing number of Brucella ceti isolates has been reported in members of the two suborders of cetaceans: Mysticeti and Odontoceti. Serological surveys have shown that cetacean brucellosis may be distributed worldwide in the oceans. Although all B. ceti isolates have been included within the same species, three different groups have been recognized according to their preferred host, bacteriological properties, and distinct genetic traits: B. ceti dolphin type, B. ceti porpoise type, and B. ceti human type. It seems that B. ceti porpoise type is more closely related to B. ceti human isolates and B. pinnipedialis group, while B. ceti dolphin type seems ancestral to them. Based on comparative phylogenetic analysis, it is feasible that the B. ceti ancestor radiated in a terrestrial artiodactyl host close to the Raoellidae family about 58 million years ago. The more likely mode of transmission of B. ceti seems to be through sexual intercourse, maternal feeding, aborted fetuses, placental tissues, vertical transmission from mother to the fetus or through fish or helminth reservoirs. The B. ceti dolphin and porpoise types seem to display variable virulence in land animal models and low infectivity for humans. However, brucellosis in some dolphins and porpoises has been demonstrated to be a severe chronic disease, displaying significant clinical and pathological signs related to abortions, male infertility, neurobrucellosis, cardiopathies, bone and skin lesions, strandings, and death.
Keywords: Brucella; Brucella ceti; brucellosis; cetacean; dolphin; marine brucellosis; porpoise; whale.
Figures


Similar articles
-
Brucella ceti infection in dolphins from the Western Mediterranean sea.BMC Vet Res. 2014 Sep 17;10:206. doi: 10.1186/s12917-014-0206-7. BMC Vet Res. 2014. PMID: 25224818 Free PMC article.
-
MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis.BMC Microbiol. 2009 Jul 20;9:145. doi: 10.1186/1471-2180-9-145. BMC Microbiol. 2009. PMID: 19619320 Free PMC article.
-
Wildlife reservoirs of brucellosis: Brucella in aquatic environments.Rev Sci Tech. 2013 Apr;32(1):89-103. doi: 10.20506/rst.32.1.2194. Rev Sci Tech. 2013. PMID: 23837368
-
A review of Brucella infection in marine mammals, with special emphasis on Brucella pinnipedialis in the hooded seal (Cystophora cristata).Vet Res. 2011 Aug 5;42(1):93. doi: 10.1186/1297-9716-42-93. Vet Res. 2011. PMID: 21819589 Free PMC article. Review.
-
[Zoonotic potential of brucellosis in marine mammals].Med Trop Sante Int. 2024 Feb 19;4(1):mtsi.v4i1.2024.489. doi: 10.48327/mtsi.v4i1.2024.489. eCollection 2024 Mar 31. Med Trop Sante Int. 2024. PMID: 38846127 Free PMC article. Review. French.
Cited by
-
Core Genome Multilocus Sequence Typing Scheme for Improved Characterization and Epidemiological Surveillance of Pathogenic Brucella.J Clin Microbiol. 2022 Aug 17;60(8):e0031122. doi: 10.1128/jcm.00311-22. Epub 2022 Jul 19. J Clin Microbiol. 2022. PMID: 35852343 Free PMC article.
-
Marine Mammal Brucella Reference Strains Are Attenuated in a BALB/c Mouse Model.PLoS One. 2016 Mar 9;11(3):e0150432. doi: 10.1371/journal.pone.0150432. eCollection 2016. PLoS One. 2016. PMID: 26959235 Free PMC article.
-
Brucella ceti infection in dolphins from the Western Mediterranean sea.BMC Vet Res. 2014 Sep 17;10:206. doi: 10.1186/s12917-014-0206-7. BMC Vet Res. 2014. PMID: 25224818 Free PMC article.
-
Brucella Genetic Variability in Wildlife Marine Mammals Populations Relates to Host Preference and Ocean Distribution.Genome Biol Evol. 2017 Jul 1;9(7):1901-1912. doi: 10.1093/gbe/evx137. Genome Biol Evol. 2017. PMID: 28854602 Free PMC article.
-
Serological Diagnosis of Brucella Infection in Cetaceans by Rapid Serum Agglutination Test and Competitive ELISA with Brucella abortus and Brucella ceti as Antigens.Pathogens. 2025 Jan 2;14(1):26. doi: 10.3390/pathogens14010026. Pathogens. 2025. PMID: 39860987 Free PMC article.
References
-
- Åkerstrom B., Brodin T., Reis K., Björck L. (1985). Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J. Immunol. 135, 2589–2592 - PubMed
-
- Alekseev A., Yu E., Rozanova I., Ustinova E. N., Tumanov Yu. I., Kuvshinova I. N., Shestopalov A. M. (2007). The prevalence of antibodies to morbilliviruses, Brucella, and Toxoplasma in the Black Sea bottlenose dolphin Tursiops truncatus ponticus maintained in captivity. Russ. J. Mar. Biol. 33, 425–42810.1134/S1063074007060107 - DOI
-
- Alton G. G. (1990). “Brucella melitensis,” in Animal Brucellosis, eds Nielsen K. H., Duncan J. R. (Boca Raton, FL: CRC Press; ), 383–409
-
- Alton G. G., Jones L. M., Pietz D. E. (1975). Bacteriological Methods. Laboratory Techniques in Brucellosis. Geneva: World Health Organization - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

