Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism?
- PMID: 22919617
- PMCID: PMC3417527
- DOI: 10.3389/fcimb.2012.00025
Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism?
Abstract
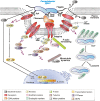
Campylobacter jejuni, a spiral-shaped Gram-negative pathogen, is a highly frequent cause of gastrointestinal foodborne illness in humans worldwide. Clinical outcome of C. jejuni infections ranges from mild to severe diarrheal disease, and some other complications including reactive arthritis and Guillain-Barré syndrome. This review article highlights various C. jejuni pathogenicity factors, host cell determinants, and proposed signaling mechanisms involved in human host cell invasion and their potential role in the development of C. jejuni-mediated disease. A model is presented which outlines the various important interactions of C. jejuni with the intestinal epithelium, and we discuss the pro's and con's for the "zipper" over the "trigger" mechanism of invasion. Future work should clarify the contradictory role of some previously identified factors, and should identify and characterize novel virulence determinants, which are crucial to provide fresh insights into the diversity of strategies employed by this pathogen to cause disease.
Keywords: cellular invasion; molecular pathogenesis; signaling; virulence.
Figures

References
-
- Ashgar S. S., Oldfield N. J., Woolridge K. G., Jones M. A., Irving G. J., Turner D. P., Ala’Aldeen D. A. (2007). CapA, an autotransporter protein of Campylobacter jejuni mediates association with human epithelial cells and colonization of the chicken gut. J. Bacteriol. 189, 1856–186510.1128/JB.01427-06 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

