The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells
- PMID: 22919623
- PMCID: PMC3417660
- DOI: 10.3389/fcimb.2012.00031
The Campylobacter jejuni CiaC virulence protein is secreted from the flagellum and delivered to the cytosol of host cells
Abstract
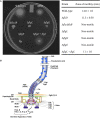
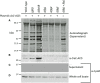
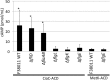
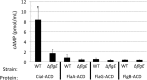
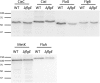
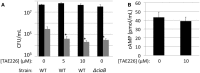
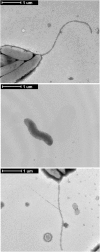
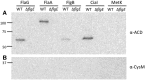
Campylobacter jejuni is a leading cause of bacterial gastroenteritis worldwide. Acute C. jejuni-mediated disease (campylobacteriosis) involves C. jejuni invasion of host epithelial cells using adhesins (e.g., CadF and FlpA) and secreted proteins [e.g., the Campylobacter invasion antigens (Cia)]. The genes encoding the Cia proteins are up-regulated upon co-culture of C. jejuni with epithelial cells. One of the Cia proteins, CiaC, is required for maximal invasion of host cells by C. jejuni. Previous work has also revealed that CiaC is, in part, responsible for host cell cytoskeletal rearrangements that result in membrane ruffling. This study was performed to test the hypothesis that CiaC is delivered to the cytosol of host cells. To detect the delivery of CiaC into cultured epithelial cells, we used the adenylate cyclase domain (ACD) of Bordetella pertussis CyaA as a reporter. In this study, we found that export and delivery of the C. jejuni Cia proteins into human INT 407 epithelial cells required a functional flagellar hook complex composed of FlgE, FlgK, and FlgL. Assays performed with bacterial culture supernatants supported the hypothesis that CiaC delivery requires bacteria-host cell contact. We also found that CiaC was delivered to host cells by cell-associated (bound) bacteria, as judged by experiments performed with inhibitors that specifically target the cell signaling pathways utilized by C. jejuni for cell invasion. Interestingly, the C. jejuni flgL mutant, which is incapable of exporting and delivering the Cia proteins, did not induce INT 407 cell membrane ruffles. Complementation of the flgL mutant with plasmid-encoded flgL restored the motility and membrane ruffling. These data support the hypothesis that the C. jejuni Cia proteins, which are exported from the flagellum, are delivered to the cytosol of host cells.
Keywords: T3SS; adenylate cyclase; effector proteins; flagellum; membrane ruffling.
Figures




References
-
- Ashgar S. S., Oldfield N. J., Wooldridge K. G., Jones M. A., Irving G. J., Turner D. P., Ala'Aldeen D. A. (2007). CapA, an autotransporter protein of Campylobacter jejuni, mediates association with human epithelial cells and colonization of the chicken gut. J. Bacteriol. 189, 1856–1865 10.1128/JB.01427-06 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

