Single-cell elemental analysis of bacteria: quantitative analysis of polyphosphates in Mycobacterium tuberculosis
- PMID: 22919654
- PMCID: PMC3417655
- DOI: 10.3389/fcimb.2012.00063
Single-cell elemental analysis of bacteria: quantitative analysis of polyphosphates in Mycobacterium tuberculosis
Abstract
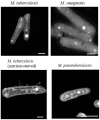
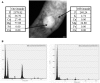
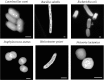
More than 1.8 million people die annually from infection with Mycobacterium tuberculosis, the causative agent of tuberculosis. The ability of M. tuberculosis to obtain and distribute micronutrients, including biometals, is known to play a role in its intracellular survival and virulence within a host. Techniques to detect elemental distributions within M. tuberculosis cells have previously been limited to bulk detection methods or low-resolution analyses. Here, we present a method for determining the elemental distribution within M. tuberculosis on a single-cell level, at high (individual nanometer) resolution, using scanning transmission electron microscopy (STEM) in concert with energy-dispersive X-ray spectroscopy (EDS). Results revealed the presence of large polyphosphate granules in all strains of Mycobacteria tested. These persisted even through starvation conditions, and might play a role connected to elemental homeostasis in M. tuberculosis. Associated with the polyphosphate granules were micronutrients such as calcium and magnesium. In addition, we expanded the technique beyond Mycobacteria to show that STEM and EDS could be used as a simple screen to detect the presence or absence of concentrated elements on a single-cell level within all six other bacterial types tested, with minimal processing to the bacteria. Overall, we believe that this technique represents a first step in developing a better understanding of the role that components of the intracellular milieu, including polyphosphates and biometals, play in the pathogenesis of M. tuberculosis, with potential future applications for in vivo analysis.
Keywords: elements; metals; scanning transmission electron microscopy; tuberculosis.
Figures
Similar articles
-
Discovery and Characterization of Iron Sulfide and Polyphosphate Bodies Coexisting in Archaeoglobus fulgidus Cells.Archaea. 2016 Apr 19;2016:4706532. doi: 10.1155/2016/4706532. eCollection 2016. Archaea. 2016. PMID: 27194953 Free PMC article.
-
Polyphosphate storage during sporulation in the gram-negative bacterium Acetonema longum.J Bacteriol. 2013 Sep;195(17):3940-6. doi: 10.1128/JB.00712-13. J Bacteriol. 2013. PMID: 23813732 Free PMC article.
-
Stability of enhanced biological phosphorus removal and composition of polyphosphate granules.Water Res. 2001 Sep;35(13):3190-6. doi: 10.1016/s0043-1354(01)00025-2. Water Res. 2001. PMID: 11487116
-
Microscopy Hacks: development of various techniques to assist quantitative nanoanalysis and advanced electron microscopy.Microscopy (Oxf). 2013 Apr;62(2):217-41. doi: 10.1093/jmicro/dfs085. Epub 2013 Mar 20. Microscopy (Oxf). 2013. PMID: 23515525 Review.
-
[Polyphosphate and its physiological function in Mycobacteria - A review].Wei Sheng Wu Xue Bao. 2016 Dec 4;56(12):1840-6. Wei Sheng Wu Xue Bao. 2016. PMID: 29741848 Review. Chinese.
Cited by
-
Calling all hosts: Bacterial communication in situ.Chem. 2017 Mar 9;2(3):334-358. doi: 10.1016/j.chempr.2017.02.001. Chem. 2017. PMID: 28948238 Free PMC article.
-
Ultrastructural insights into cellular organization, energy storage and ribosomal dynamics of an ammonia-oxidizing archaeon from oligotrophic oceans.Front Microbiol. 2024 Apr 26;15:1367658. doi: 10.3389/fmicb.2024.1367658. eCollection 2024. Front Microbiol. 2024. PMID: 38737410 Free PMC article.
-
Reentrant DNA shells tune polyphosphate condensate size.Nat Commun. 2024 Oct 26;15(1):9258. doi: 10.1038/s41467-024-53469-x. Nat Commun. 2024. PMID: 39462120 Free PMC article.
-
Mycobacterium tuberculosis and Copper: A Newly Appreciated Defense against an Old Foe?J Biol Chem. 2015 Jul 31;290(31):18962-6. doi: 10.1074/jbc.R115.640193. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055711 Free PMC article. Review.
-
Reentrant DNA shells tune polyphosphate condensate size.bioRxiv [Preprint]. 2023 Sep 15:2023.09.13.557044. doi: 10.1101/2023.09.13.557044. bioRxiv. 2023. Update in: Nat Commun. 2024 Oct 26;15(1):9258. doi: 10.1038/s41467-024-53469-x. PMID: 37745474 Free PMC article. Updated. Preprint.
References
-
- Albrecht R., Olorundare O., liver J., Meyer D. (2011). Nanoparticle labels for co-localization and correlative imaging at high spatial resolution. Microsc. Microanal. 17, 356–35710.1017/S1431927611002650 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

