Development of a murine nose-only inhalation model of influenza: comparison of disease caused by instilled and inhaled A/PR/8/34
- PMID: 22919665
- PMCID: PMC3417508
- DOI: 10.3389/fcimb.2012.00074
Development of a murine nose-only inhalation model of influenza: comparison of disease caused by instilled and inhaled A/PR/8/34
Abstract
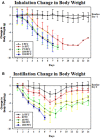
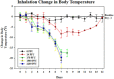
Influenza continues to cause widespread disease and death during winter months. In preclinical studies to evaluate the potential efficacy of drugs and vaccines, influenza challenge virus is usually instilled into the noses of animals in the form of large liquid drops. Since inhalation of aerosolized influenza is commonly associated with human transmission, instillation of challenge virus raises uncertainty about the applicability of results. In order to compare the challenge methods, we established conditions to generate influenza aerosols with a mass median aerodynamic diameter (MMAD) of 1 μm that were delivered to mice in a nose-only inhalation system. In this report, we describe the system and compare the 50% lethal dose (LD(50)) of instilled and inhaled A/PR/8/34 (PR8) in BALB/c mice. The estimated LD(50) for inhaled virus was 8.7 plaque forming units (PFU) and the mean time to death was 7.7 days, whereas the estimated LD(50) for instilled virus was 51.6 PFU and the mean time to death was 8.2 days. Our results show that mice are more sensitive to inhaled virus than virus delivered by intranasal instillation. The murine nose-only inhalation model of influenza infection can be used to infect large numbers of animals simultaneously with well-characterized, homogenous PR8 bioaerosol in a controlled and reproducible manner. This model provides the means to evaluate the efficacy of drug and vaccine candidates against the relevant route of challenge, thereby providing data that may better predict clinical outcome.
Keywords: aerosol; influenza; inhalation; instillation; mouse.
Figures



References
-
- Alford R. H., Kasel J. A., Gerone P. J., Knight V. (1966). Human influenza resulting from aerosol inhalation. Proc. Soc. Exp. Biol. Med. 122, 800–804 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

