Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis
- PMID: 22919683
- PMCID: PMC3417644
- DOI: 10.3389/fcimb.2012.00092
Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis
Abstract
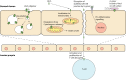
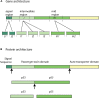
More than 50% of the world's population is infected with Helicobacter pylori (H. pylori). Chronic infection with this Gram-negative pathogen is associated with the development of peptic ulcers and is linked to an increased risk of gastric cancer. H. pylori secretes many proteinaceous factors that are important for initial colonization and subsequent persistence in the host stomach. One of the major protein toxins secreted by H. pylori is the Vacuolating cytotoxin A (VacA). After secretion from the bacteria via a type V autotransport secretion system, the 88 kDa VacA toxin (comprised of the p33 and p55 subunits) binds to host cells and is internalized, causing severe "vacuolation" characterized by the accumulation of large vesicles that possess hallmarks of both late endosomes and early lysosomes. The development of "vacuoles" has been attributed to the formation of VacA anion-selective channels in membranes. Apart from its vacuolating effects, it has recently become clear that VacA also directly affects mitochondrial function. Earlier studies suggested that the p33 subunit, but not the p55 subunit of VacA, could enter mitochondria to modulate organelle function. This raised the possibility that a mechanism separate from pore formation may be responsible for the effects of VacA on mitochondria, as crystallography studies and structural modeling predict that both subunits are required for a physiologically stable pore. It has also been suggested that the mitochondrial effects observed are due to indirect effects on pro-apoptotic proteins and direct effects on mitochondrial morphology-related processes. Other studies have shown that both the p55 and p33 subunits can indeed be efficiently imported into mammalian-derived mitochondria raising the possibility that they could re-assemble to form a pore. Our review summarizes and consolidates the recent advances in VacA toxin research, with focus on the outstanding controversies in the field and the key remaining questions that need to be addressed.
Keywords: Helicobacter pylori; VacA.
Figures

References
-
- Atherton J. C., Cao P., Peek R. M. Jr., Tummuru M. K., Blaser M. J., Cover T. L. (1995). Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270, 17771–17777 10.1074/jbc.270.30.17771 - DOI - PubMed
-
- Backert S., Ziska E., Brinkmann V., Zimny-Arndt U., Fauconnier A., Jungblut P. R., Naumann M., Meyer T. F. (2000). Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2, 155–164 10.1046/j.1462-5822.2000.00043.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

