Choice of inbred rat strain impacts lethality and disease course after respiratory infection with Rift Valley Fever Virus
- PMID: 22919694
- PMCID: PMC3417668
- DOI: 10.3389/fcimb.2012.00105
Choice of inbred rat strain impacts lethality and disease course after respiratory infection with Rift Valley Fever Virus
Abstract
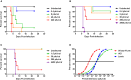
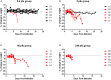
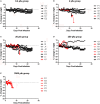
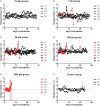
Humans infected with Rift Valley Fever Virus (RVFV) generally recover after a febrile illness; however, a proportion of patients progress to a more severe clinical outcome such as hemorrhagic fever or meningoencephalitis. RVFV is naturally transmitted to livestock and humans by mosquito bites, but it is also infectious through inhalational exposure, making it a potential bioterror weapon. To better understand the disease caused by inhalation of RVFV, Wistar-Furth, ACI, or Lewis rats were exposed to experimental aerosols containing virulent RVFV. Wistar-Furth rats developed a rapidly progressing lethal hepatic disease after inhalational exposure; ACI rats were 100-fold less susceptible and developed fatal encephalitis after infection. Lewis rats, which do not succumb to parenteral inoculation with RVFV, developed fatal encephalitis after aerosol infection. RVFV was found in the liver, lung, spleen, heart, kidney and brain of Wistar Furth rats that succumbed after aerosol exposure. In contrast, RVFV was found only in the brains of ACI or Lewis rats that succumbed after aerosol exposure. Lewis rats that survived s.c. infection were not protected against subsequent re-challenge by aerosol exposure to the homologous virus. This is the first side-by-side comparison of the lethality and pathogenesis of RVFV in three rat strains after aerosol exposure and the first step toward developing a rodent model suitable for use under the FDA Animal Rule to test potential vaccines and therapeutics for aerosol exposure to RVFV.
Keywords: LD50; Rift Valley Fever Virus; aerosol exposure; inbred rat strain; respiratory infection.
Figures


References
-
- Abdel-Wahab K. S., El Baz L. M., El-Tayeb E. M., Omar H., Ossman M. A., Yasin W. (1978). Rift Valley Fever virus infections in Egypt: pathological and virological findings in man. Trans. R. Soc. Trop. Med. Hyg. 72, 392–396 - PubMed
-
- Anderson G. W., Jr., Lee J. O., Anderson A. O., Powell N., Mangiafico J. A., Meadors G. (1991a). Efficacy of a Rift Valley fever virus vaccine against an aerosol infection in rats. Vaccine 9, 710–714 - PubMed
-
- Anderson G. W., Jr., Rosebrock J. A., Johnson A. J., Jennings G. B., Peters C. J. (1991b). Infection of inbred rat strains with Rift Valley fever virus: development of a congenic resistant strain and observations on age-dependence of resistance. Am. J. Trop. Med. Hyg. 44, 475–480 - PubMed
-
- Anderson G. W., Jr., Slone T. W., Jr., Peters C. J. (1987). Pathogenesis of Rift Valley fever virus (RVFV) in inbred rats. Microb. Pathog. 2, 283–293 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

