Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro
- PMID: 22920231
- PMCID: PMC3493356
- DOI: 10.1186/1472-6882-12-138
Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro
Abstract
Background: Doxorubicin (DOX) is an effective antineoplastic drug; however, clinical use of DOX is limited by its dose-dependent cardiotoxicity. It is well known that reactive oxygen species (ROS) play a vital role in the pathological process of DOX-induced cardiotoxicity. For this study, we evaluated the protective effects of guggulsterone (GS), a steroid obtained from myrrh, to determine its preliminary mechanisms in defending against DOX-induced cytotoxicity in H9C2 cells.
Methods: In this study, we used a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay, lactate dehydrogenase (LDH) release measurements, and Hoechst 33258 staining to evaluate the protective effect of GS against DOX-induced cytotoxicity in H9C2 cells. In addition, we observed the immunofluorescence of intracellular ROS and measured lipid peroxidation, caspase-3 activity, and apoptosis-related proteins by using Western blotting.
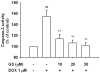
Results: The MTT assay and LDH release showed that treatment using GS (1-30 μM) did not cause cytotoxicity. Furthermore, GS inhibited DOX (1 μM)-induced cytotoxicity in a concentration-dependent manner. Hoechst 33258 staining showed that GS significantly reduced DOX-induced apoptosis and cell death. Using GS at a dose of 10-30 μM significantly reduced intracellular ROS and the formation of MDA in the supernatant of DOX-treated H9C2 cells and suppressed caspase-3 activity to reference levels. In immunoblot analysis, pretreatment using GS significantly reversed DOX-induced decrease of PARP, caspase-3 and bcl-2, and increase of bax, cytochrome C release, cleaved-PARP and cleaved-caspase-3. In addition, the properties of DOX-induced cancer cell (DLD-1 cells) death did not interfere when combined GS and DOX.
Conclusion: These data provide considerable evidence that GS could serve as a novel cardioprotective agent against DOX-induced cardiotoxicity.
Figures

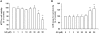
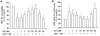


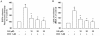



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

