c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration
- PMID: 22920255
- PMCID: PMC3657176
- DOI: 10.1016/j.neuron.2012.06.021
c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration
Abstract
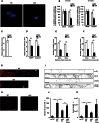
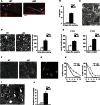
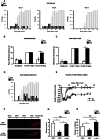
The radical response of peripheral nerves to injury (Wallerian degeneration) is the cornerstone of nerve repair. We show that activation of the transcription factor c-Jun in Schwann cells is a global regulator of Wallerian degeneration. c-Jun governs major aspects of the injury response, determines the expression of trophic factors, adhesion molecules, the formation of regeneration tracks and myelin clearance and controls the distinctive regenerative potential of peripheral nerves. A key function of c-Jun is the activation of a repair program in Schwann cells and the creation of a cell specialized to support regeneration. We show that absence of c-Jun results in the formation of a dysfunctional repair cell, striking failure of functional recovery, and neuronal death. We conclude that a single glial transcription factor is essential for restoration of damaged nerves, acting to control the transdifferentiation of myelin and Remak Schwann cells to dedicated repair cells in damaged tissue.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
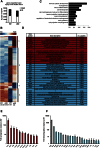
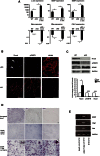
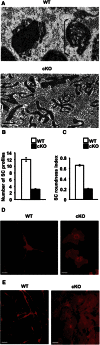
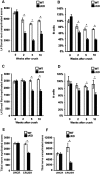
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. - PubMed
-
- Adameyko I., Lallemend F., Aquino J.B., Pereira J.A., Topilko P., Müller T., Fritz N., Beljajeva A., Mochii M., Liste I. Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell. 2009;139:366–379. - PubMed
-
- Chen Z.L., Yu W.M., Strickland S. Peripheral regeneration. Annu. Rev. Neurosci. 2007;30:209–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT088646MA/WT_/Wellcome Trust/United Kingdom
- G0600967/MRC_/Medical Research Council/United Kingdom
- 15679/CRUK_/Cancer Research UK/United Kingdom
- S20299/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- B/D009537/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
