Cation clock permits distinction between the mechanisms of α- and β-O- and β-C-glycosylation in the mannopyranose series: evidence for the existence of a mannopyranosyl oxocarbenium ion
- PMID: 22920536
- PMCID: PMC3448556
- DOI: 10.1021/ja307266n
Cation clock permits distinction between the mechanisms of α- and β-O- and β-C-glycosylation in the mannopyranose series: evidence for the existence of a mannopyranosyl oxocarbenium ion
Abstract
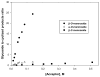
The use of a cationic cyclization reaction as a probe of the glycosylation mechanism has been developed and applied to the 4,6-O-benzylidene-protected mannopyranoside system. Cyclization results in the formation of both cis- and trans-fused tricyclic systems, invoking an intermediate glycosyl oxocarbenium ion reacting through a boat conformation. Competition reactions with isopropanol and trimethyl(methallyl)silane are interpreted as indicating that β-O-mannosylation proceeds via an associative S(N)2-like mechanism, whereas α-O-mannosylation and β-C-mannosylation are dissociative and S(N)1-like. Relative rate constants for reactions going via a common intermediate can be estimated.
Figures

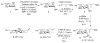

References
-
- Saito K, Ueoka K, Matsumoto K, Suga S, Nokami T, Yoshida J-i. Angew Chem Int Ed. 2011;50:5153–5156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

