Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration
- PMID: 22920724
- PMCID: PMC3580473
- DOI: 10.1186/scrt126
Adipose stem cells can secrete angiogenic factors that inhibit hyaline cartilage regeneration
Abstract
Introduction: Adipose stem cells (ASCs) secrete many trophic factors that can stimulate tissue repair, including angiogenic factors, but little is known about how ASCs and their secreted factors influence cartilage regeneration. Therefore, the aim of this study was to determine the effects ASC-secreted factors have in repairing chondral defects.
Methods: ASCs isolated from male Sprague Dawley rats were cultured in monolayer or alginate microbeads supplemented with growth (GM) or chondrogenic medium (CM). Subsequent co-culture, conditioned media, and in vivo cartilage defect studies were performed.
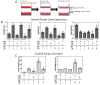
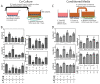
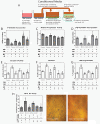
Results: ASC monolayers and microbeads cultured in CM had decreased FGF-2 gene expression and VEGF-A secretion compared to ASCs cultured in GM. Chondrocytes co-cultured with GM-cultured ASCs for 7 days had decreased mRNAs for col2, comp, and runx2. Chondrocytes treated for 12 or 24 hours with conditioned medium from GM-cultured ASCs had reduced sox9, acan, and col2 mRNAs; reduced proliferation and proteoglycan synthesis; and increased apoptosis. ASC-conditioned medium also increased endothelial cell tube lengthening whereas conditioned medium from CM-cultured ASCs had no effect. Treating ASCs with CM reduced or abolished these deleterious effects while adding a neutralizing antibody for VEGF-A eliminated ASC-conditioned medium induced chondrocyte apoptosis and restored proteoglycan synthesis. FGF-2 also mitigated the deleterious effects VEGF-A had on chondrocyte apoptosis and phenotype. When GM-grown ASC pellets were implanted in 1 mm non-critical hyaline cartilage defects in vivo, cartilage regeneration was inhibited as evaluated by radiographic and equilibrium partitioning of an ionic contrast agent via microCT imaging. Histology revealed that defects with GM-cultured ASCs had no tissue ingrowth from the edges of the defect whereas empty defects and defects with CM-grown ASCs had similar amounts of neocartilage formation.
Conclusions: ASCs must be treated to reduce the secretion of VEGF-A and other factors that inhibit cartilage regeneration, which can significantly influence how ASCs are used for repairing hyaline cartilage.
Figures
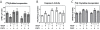

Similar articles
-
Tailoring adipose stem cell trophic factor production with differentiation medium components to regenerate chondral defects.Tissue Eng Part A. 2013 Jun;19(11-12):1451-64. doi: 10.1089/ten.TEA.2012.0233. Epub 2013 Mar 28. Tissue Eng Part A. 2013. PMID: 23350662 Free PMC article.
-
Influence of chondrocytes on the chondrogenic differentiation of adipose stem cells.Tissue Eng Part A. 2010 Dec;16(12):3569-77. doi: 10.1089/ten.TEA.2010.0218. Epub 2010 Aug 28. Tissue Eng Part A. 2010. PMID: 20597811
-
An effective approach to cartilage regeneration using antler stem cell-conditioned medium.Sci Rep. 2025 Jul 31;15(1):27971. doi: 10.1038/s41598-025-13841-3. Sci Rep. 2025. PMID: 40745014 Free PMC article.
-
Adipose-derived stem cells in cartilage regeneration: current perspectives.Regen Med. 2016 Oct;11(7):693-703. doi: 10.2217/rme-2016-0077. Epub 2016 Sep 6. Regen Med. 2016. PMID: 27599358 Review.
-
Generation of reactive oxygen species in adipose-derived stem cells: friend or foe?Expert Opin Ther Targets. 2011 Nov;15(11):1297-306. doi: 10.1517/14728222.2011.628315. Epub 2011 Oct 10. Expert Opin Ther Targets. 2011. PMID: 21981031 Free PMC article. Review.
Cited by
-
Stem cells catalyze cartilage formation by neonatal articular chondrocytes in 3D biomimetic hydrogels.Sci Rep. 2013 Dec 19;3:3553. doi: 10.1038/srep03553. Sci Rep. 2013. PMID: 24352100 Free PMC article.
-
Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair.Int J Mol Sci. 2020 Sep 24;21(19):7038. doi: 10.3390/ijms21197038. Int J Mol Sci. 2020. PMID: 32987830 Free PMC article. Review.
-
CD271-selected mesenchymal stem cells from adipose tissue enhance cartilage repair and are less angiogenic than plastic adherent mesenchymal stem cells.Sci Rep. 2019 Feb 28;9(1):3194. doi: 10.1038/s41598-019-39715-z. Sci Rep. 2019. PMID: 30816233 Free PMC article.
-
Adipose stem cell microbeads as production sources for chondrogenic growth factors.J Stem Cells Regen Med. 2014 Nov 28;10(2):38-48. doi: 10.46582/jsrm.1002007. eCollection 2014. J Stem Cells Regen Med. 2014. PMID: 25705097 Free PMC article.
-
Non-contact Coculture Reveals a Comprehensive Response of Chondrocytes Induced by Mesenchymal Stem Cells Through Trophic Secretion.Tissue Eng Regen Med. 2017 Sep 27;15(1):37-48. doi: 10.1007/s13770-017-0084-8. eCollection 2018 Feb. Tissue Eng Regen Med. 2017. PMID: 30603533 Free PMC article.
References
-
- Gimble JM, Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Curr Top Dev Biol. 2003;58:137–160. - PubMed
-
- Macchiarini P, Birchall M, Hollander A, Mantero S, Conconi MT. Clinical transplantation of a tissue-engineered airway Authors' reply. Lancet. 2009;373:718–719. - PubMed
-
- Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24:347–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

