Firefly luciferase in chemical biology: a compendium of inhibitors, mechanistic evaluation of chemotypes, and suggested use as a reporter
- PMID: 22921073
- PMCID: PMC3449281
- DOI: 10.1016/j.chembiol.2012.07.015
Firefly luciferase in chemical biology: a compendium of inhibitors, mechanistic evaluation of chemotypes, and suggested use as a reporter
Abstract
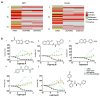
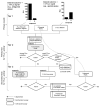
Firefly luciferase (FLuc) is frequently used as a reporter in high-throughput screening assays, owing to the exceptional sensitivity, dynamic range, and rapid measurement that bioluminescence affords. However, interaction of small molecules with FLuc has, to some extent, confounded its use in chemical biology and drug discovery. To identify and characterize chemotypes interacting with FLuc, we determined potency values for 360,864 compounds found in the NIH Molecular Libraries Small Molecule Repository, available in PubChem. FLuc inhibitory activity was observed for 12% of this library with discernible SAR. Characterization of 151 inhibitors demonstrated a variety of inhibition modes, including FLuc-catalyzed formation of multisubstrate adduct enzyme inhibitor complexes. As in some cell-based FLuc reporter assays, compounds acting as FLuc inhibitors yield paradoxical luminescence increases, thus data on compounds acquired from FLuc-dependent assays require careful analysis as described here.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflict of interest.
Figures




References
-
- Auld DS, Southall NT, Jadhav A, Johnson RL, Diller DJ, Simeonov A, Austin CP, Inglese J. Characterization of chemical libraries for luciferase inhibitory activity. J Med Chem. 2008a;51:2372–2386. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

