Temperature field reconstruction for minimally invasive cryosurgery with application to wireless implantable temperature sensors and/or medical imaging
- PMID: 22921369
- PMCID: PMC3529162
- DOI: 10.1016/j.cryobiol.2012.08.001
Temperature field reconstruction for minimally invasive cryosurgery with application to wireless implantable temperature sensors and/or medical imaging
Abstract
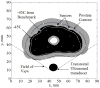
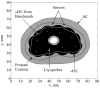
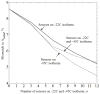
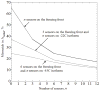
There is an undisputed need for temperature-field reconstruction during minimally invasive cryosurgery. The current line of research focuses on developing miniature, wireless, implantable, temperature sensors to enable temperature-field reconstruction in real time. This project combines two parallel efforts: (i) to develop the hardware necessary for implantable sensors, and (ii) to develop mathematical techniques for temperature-field reconstruction in real time-the subject matter of the current study. In particular, this study proposes an approach for temperature-field reconstruction combining data obtained from medical imaging, cryoprobe-embedded sensors, and miniature, wireless, implantable sensors, the development of which is currently underway. This study discusses possible strategies for laying out implantable sensors and approaches for data integration. In particular, prostate cryosurgery is presented as a developmental model and a two-dimensional proof-of-concept is discussed. It is demonstrated that the lethal temperature can be predicted to a significant degree of certainty with implantable sensors and the technique proposed in the current study, a capability that is yet unavailable.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures






Similar articles
-
A new method for temperature-field reconstruction during ultrasound-monitored cryosurgery using potential-field analogy.Cryobiology. 2016 Feb;72(1):69-77. doi: 10.1016/j.cryobiol.2015.10.153. Epub 2015 Nov 14. Cryobiology. 2016. PMID: 26586098 Free PMC article.
-
Characterization of a CMOS sensing core for ultra-miniature wireless implantable temperature sensors with application to cryomedicine.Med Eng Phys. 2014 Sep;36(9):1191-6. doi: 10.1016/j.medengphy.2014.05.002. Epub 2014 Jul 4. Med Eng Phys. 2014. PMID: 25001173 Free PMC article.
-
MR imaging assisted temperature calculations during cryosurgery.Magn Reson Imaging. 1994;12(7):1021-31. doi: 10.1016/0730-725x(94)91233-m. Magn Reson Imaging. 1994. PMID: 7997089
-
Future directions for cryosurgery computer treatment planning.Urology. 2002 Aug;60(2 Suppl 1):50-5. doi: 10.1016/s0090-4295(02)01684-9. Urology. 2002. PMID: 12206848 Review.
-
The evolution and state of modern technology for prostate cryosurgery.Urology. 2002 Aug;60(2 Suppl 1):26-33. doi: 10.1016/s0090-4295(02)01681-3. Urology. 2002. PMID: 12206845 Review.
Cited by
-
A new method for temperature-field reconstruction during ultrasound-monitored cryosurgery using potential-field analogy.Cryobiology. 2016 Feb;72(1):69-77. doi: 10.1016/j.cryobiol.2015.10.153. Epub 2015 Nov 14. Cryobiology. 2016. PMID: 26586098 Free PMC article.
-
Defeating Cancers' Adaptive Defensive Strategies Using Thermal Therapies: Examining Cancer's Therapeutic Resistance, Ablative, and Computational Modeling Strategies as a means for Improving Therapeutic Outcome.Technol Cancer Res Treat. 2018 Jan 1;17:1533033818762207. doi: 10.1177/1533033818762207. Technol Cancer Res Treat. 2018. PMID: 29566612 Free PMC article. Review.
-
Characterization of a CMOS sensing core for ultra-miniature wireless implantable temperature sensors with application to cryomedicine.Med Eng Phys. 2014 Sep;36(9):1191-6. doi: 10.1016/j.medengphy.2014.05.002. Epub 2014 Jul 4. Med Eng Phys. 2014. PMID: 25001173 Free PMC article.
-
Simulation and evaluation of freeze-thaw cryoablation scenarios for the treatment of cardiac arrhythmias.Biomed Eng Online. 2015 Feb 18;14:12. doi: 10.1186/s12938-015-0005-9. Biomed Eng Online. 2015. PMID: 25886498 Free PMC article.
References
-
- Baust JG, Gage AA, Klossner D, Clarke D, Miller R, Cohen J, Katz A, Polascik T, Clarke H, Baust JM. Issues critical to the successful application of cryosurgical ablation of the prostate. Technol Cancer Res Treat. 2007;6(2):97–109. - PubMed
-
- Best Practice Policy Statement on Cryosurgery for the Treatment of Localized Prostate Cancar. American Urological Association; www.auanet.org, retrieved 1/15/2012.
-
- Charny KC. Mathematical models of bioheat transfer. In: Hartnett JP, Irvine TF, Cho YI, editors. Advances in Heat Transfer. Academic Press; 1992. pp. 19–156.
-
- Cooper I, Lee A. Cryostatic congelation: a system for producing a limited controlled region of cooling or freezing of biological tissue. J Nerv Ment Dis. 1961;133:259–63. - PubMed
-
- Cytron S, Paz A, Kravchick S, Shumalinski D, Moore J. Active Rectal Wall Protection Using Direct Transperineal Cryo-Needles for Histologically Proven Prostate Adenocarcinomas. European Urology. 2003;44:315–321. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

