Fibroblast growth factor 23: state of the field and future directions
- PMID: 22921867
- PMCID: PMC3502714
- DOI: 10.1016/j.tem.2012.07.002
Fibroblast growth factor 23: state of the field and future directions
Abstract
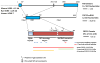
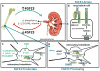
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone that regulates and is regulated by blood levels of phosphate and active vitamin D. Post-translational glycosylation by the enzyme GALNT3 and subsequent processing by furin have been demonstrated to be a regulated process that plays a role in regulating FGF23 levels. In physiologic states, FGF23 signaling is mediated by an FGF receptor and the coreceptor, Klotho. Recent work identifying a role for iron/hypoxia pathways in FGF23 physiology and their implications are discussed. Beyond its importance in primary disorders of mineral metabolism, recent work implicates FGF23 in renal disease-associated morbidity, as well as possible roles in cardiovascular disease and skeletal fragility.
Published by Elsevier Ltd.
Conflict of interest statement
Conflicts: The authors have no financial or ethical conflicts
Figures

References
-
- Prader A, et al. Rickets following bone tumor. Helvetica paediatrica acta. 1959;14:554–565. - PubMed
-
- Meyer RA, Jr, et al. Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res. 1989;4:493–500. - PubMed
-
- ADHR C. Nat Genet. Vol. 26. The ADHR Consortium; 2000. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23; pp. 345–348. - PubMed
-
- Weber TJ, et al. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

