Chemosensory behaviors of parasites
- PMID: 22921895
- PMCID: PMC5663455
- DOI: 10.1016/j.pt.2012.07.004
Chemosensory behaviors of parasites
Abstract
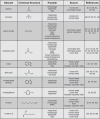
Many multicellular parasites seek out hosts by following trails of host-emitted chemicals. Host seeking is a characteristic of endoparasites such as parasitic worms as well as of ectoparasites such as mosquitoes and ticks. For host location, many of these parasites use CO(2), a respiration byproduct, in combination with host-specific chemicals. Recent work has begun to elucidate the behavioral responses of parasites to CO(2) and other host chemicals, and to unravel the mechanisms of these responses. Here we discuss recent findings that have greatly advanced our understanding of the chemosensory behaviors of host-seeking parasites. We focus primarily on well-studied parasites such as nematodes and insects, but also note broadly relevant findings in a few less well studied parasites.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Sasser JN, Freckman DW. A world perspective on nematology: the role of the society. In: Veech JA, Dickerson DW, editors. Vistas on Nematology. Society of Nematologists; Hyatsville, MD: 1987. pp. 7–14.
-
- Nguyen KB, et al. Steinernematidae: species descriptions. In: Nguyen KB, Hunt DJ, editors. Entomopathogenic nematodes: Systematics, phylogeny, and bacterial symbionts. Brill; Leiden: 2007. pp. 121–609. (Nematology monographs and perspectives).
-
- Rasmann S, et al. Ecology and evolution of soil nematode chemotaxis. J Chem Ecol 2012 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

