IKAP expression levels modulate disease severity in a mouse model of familial dysautonomia
- PMID: 22922231
- PMCID: PMC3490515
- DOI: 10.1093/hmg/dds354
IKAP expression levels modulate disease severity in a mouse model of familial dysautonomia
Abstract
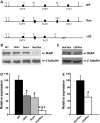
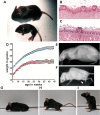
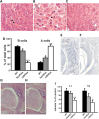
Hereditary sensory and autonomic neuropathies (HSANs) encompass a group of genetically inherited disorders characterized by sensory and autonomic dysfunctions. Familial dysautonomia (FD), also known as HSAN type III, is an autosomal recessive disorder that affects 1/3600 live births in the Ashkenazi Jewish population. The disease is caused by abnormal development and progressive degeneration of the sensory and autonomic nervous systems and is inevitably fatal, with only 50% of patients reaching the age of 40. FD is caused by a mutation in intron 20 of the Ikbkap gene that results in severe reduction in the expression of its encoded protein, inhibitor of kappaB kinase complex-associated protein (IKAP). Although the mutation that causes FD was identified in 2001, so far there is no appropriate animal model that recapitulates the disorder. Here, we report the generation and characterization of the first mouse models for FD that recapitulate the molecular and pathological features of the disease. Important for therapeutic interventions is also our finding that a slight increase in IKAP levels is enough to ameliorate the phenotype and increase the life span. Understanding the mechanisms underlying FD will provide insights for potential new therapeutic interventions not only for FD, but also for other peripheral neuropathies.
Figures

Similar articles
-
Animal and cellular models of familial dysautonomia.Clin Auton Res. 2017 Aug;27(4):235-243. doi: 10.1007/s10286-017-0438-2. Epub 2017 Jun 30. Clin Auton Res. 2017. PMID: 28667575 Free PMC article. Review.
-
Sensory and autonomic deficits in a new humanized mouse model of familial dysautonomia.Hum Mol Genet. 2016 Mar 15;25(6):1116-28. doi: 10.1093/hmg/ddv634. Epub 2016 Jan 13. Hum Mol Genet. 2016. PMID: 26769677 Free PMC article.
-
Familial Dysautonomia: Mechanisms and Models.Genet Mol Biol. 2016 Oct-Dec;39(4):497-514. doi: 10.1590/1678-4685-GMB-2015-0335. Epub 2016 Aug 4. Genet Mol Biol. 2016. PMID: 27561110 Free PMC article.
-
Deletion of exon 20 of the Familial Dysautonomia gene Ikbkap in mice causes developmental delay, cardiovascular defects, and early embryonic lethality.PLoS One. 2011;6(10):e27015. doi: 10.1371/journal.pone.0027015. Epub 2011 Oct 28. PLoS One. 2011. PMID: 22046433 Free PMC article.
-
Familial dysautonomia.Muscle Nerve. 2004 Mar;29(3):352-63. doi: 10.1002/mus.10499. Muscle Nerve. 2004. PMID: 14981733 Review.
Cited by
-
Transcriptome analysis in a humanized mouse model of familial dysautonomia reveals tissue-specific gene expression disruption in the peripheral nervous system.Sci Rep. 2024 Jan 4;14(1):570. doi: 10.1038/s41598-023-51137-6. Sci Rep. 2024. PMID: 38177237 Free PMC article.
-
Animal and cellular models of familial dysautonomia.Clin Auton Res. 2017 Aug;27(4):235-243. doi: 10.1007/s10286-017-0438-2. Epub 2017 Jun 30. Clin Auton Res. 2017. PMID: 28667575 Free PMC article. Review.
-
ELP1, the Gene Mutated in Familial Dysautonomia, Is Required for Normal Enteric Nervous System Development and Maintenance and for Gut Epithelium Homeostasis.J Neurosci. 2024 Sep 11;44(37):e2253232024. doi: 10.1523/JNEUROSCI.2253-23.2024. J Neurosci. 2024. PMID: 39138000 Free PMC article.
-
Development of the Autonomic Nervous System: Clinical Implications.Semin Neurol. 2020 Oct;40(5):473-484. doi: 10.1055/s-0040-1713926. Epub 2020 Sep 14. Semin Neurol. 2020. PMID: 32927484 Free PMC article. Review.
-
ELP1 Splicing Correction Reverses Proprioceptive Sensory Loss in Familial Dysautonomia.Am J Hum Genet. 2019 Apr 4;104(4):638-650. doi: 10.1016/j.ajhg.2019.02.009. Epub 2019 Mar 21. Am J Hum Genet. 2019. PMID: 30905397 Free PMC article.
References
-
- Riley C.M., Day R.L., Greely D., Langford W.S. Central autonomic dysfunction with defective lacrimation; report of five cases. Pediatrics. 1949;3:468–478. - PubMed
-
- Axelrod F.B., Nachtigal R., Dancis J. Familial dysautonomia: diagnosis, pathogenesis and management. Adv. Pediatr. 1974;21:75–96. - PubMed
-
- Axelrod F.B. Familial dysautonomia. Muscle Nerve. 2004;29:352–363. - PubMed
-
- Pearson J., Pytel B.A. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J. Neurol. Sci. 1978;39:47–59. - PubMed
-
- Axelrod F.B., Pearson J. Congenital sensory neuropathies. Diagnostic distinction from familial dysautonomia. Am. J. Dis. Child. 1984;138:947–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

