Bone regeneration in strong porous bioactive glass (13-93) scaffolds with an oriented microstructure implanted in rat calvarial defects
- PMID: 22922251
- PMCID: PMC3508352
- DOI: 10.1016/j.actbio.2012.08.029
Bone regeneration in strong porous bioactive glass (13-93) scaffolds with an oriented microstructure implanted in rat calvarial defects
Abstract
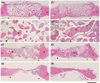
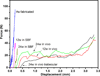
There is a need for synthetic bone graft substitutes to repair large bone defects resulting from trauma, malignancy and congenital diseases. Bioactive glass has attractive properties as a scaffold material but factors that influence its ability to regenerate bone in vivo are not well understood. In the present work, the ability of strong porous scaffolds of 13-93 bioactive glass with an oriented microstructure to regenerate bone was evaluated in vivo using a rat calvarial defect model. Scaffolds with an oriented microstructure of columnar pores (porosity=50%; pore diameter=50-150 μm) showed mostly osteoconductive bone regeneration, and new bone formation, normalized to the available pore area (volume) of the scaffolds, increased from 37% at 12 weeks to 55% at 24 weeks. Scaffolds of the same glass with a trabecular microstructure (porosity=80%; pore width=100-500 μm), used as the positive control, showed bone regeneration in the pores of 25% and 46% at 12 and 24 weeks, respectively. The brittle mechanical response of the as-fabricated scaffolds changed markedly to an elastoplastic response in vivo at both implantation times. These results indicate that both groups of 13-93 bioactive glass scaffolds could potentially be used to repair large bone defects, but scaffolds with the oriented microstructure could also be considered for the repair of loaded bone.
Copyright © 2012 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures








Similar articles
-
Investigations into the effects of scaffold microstructure on slow-release system with bioactive factors for bone repair.Front Bioeng Biotechnol. 2023 Sep 14;11:1230682. doi: 10.3389/fbioe.2023.1230682. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37781533 Free PMC article. Review.
-
Enhanced bone regeneration in rat calvarial defects implanted with surface-modified and BMP-loaded bioactive glass (13-93) scaffolds.Acta Biomater. 2013 Jul;9(7):7506-17. doi: 10.1016/j.actbio.2013.03.039. Epub 2013 Apr 6. Acta Biomater. 2013. PMID: 23567939 Free PMC article.
-
Effect of bioactive borate glass microstructure on bone regeneration, angiogenesis, and hydroxyapatite conversion in a rat calvarial defect model.Acta Biomater. 2013 Aug;9(8):8015-26. doi: 10.1016/j.actbio.2013.04.043. Epub 2013 May 2. Acta Biomater. 2013. PMID: 23643606
-
Bone regeneration in rat calvarial defects implanted with fibrous scaffolds composed of a mixture of silicate and borate bioactive glasses.Acta Biomater. 2013 Nov;9(11):9126-36. doi: 10.1016/j.actbio.2013.06.039. Epub 2013 Jul 1. Acta Biomater. 2013. PMID: 23827095
-
Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models.Acta Biomater. 2017 Oct 15;62:1-28. doi: 10.1016/j.actbio.2017.08.030. Epub 2017 Aug 24. Acta Biomater. 2017. PMID: 28844964 Review.
Cited by
-
Additive manufacturing of biomaterials.Prog Mater Sci. 2018 Apr;93:45-111. doi: 10.1016/j.pmatsci.2017.08.003. Epub 2017 Aug 26. Prog Mater Sci. 2018. PMID: 31406390 Free PMC article.
-
Biomimetic Approaches for the Design and Fabrication of Bone-to-Soft Tissue Interfaces.ACS Biomater Sci Eng. 2023 Jul 10;9(7):3810-3831. doi: 10.1021/acsbiomaterials.1c00620. Epub 2021 Nov 16. ACS Biomater Sci Eng. 2023. PMID: 34784181 Free PMC article. Review.
-
Evaluation of photobiomodulation therapy associated with guided bone regeneration in critical size defects. In vivo study.J Appl Oral Sci. 2018;26:e20170244. doi: 10.1590/1678-7757-2017-0244. Epub 2018 May 7. J Appl Oral Sci. 2018. PMID: 29742256 Free PMC article.
-
Systematical Evaluation of Mechanically Strong 3D Printed Diluted magnesium Doping Wollastonite Scaffolds on Osteogenic Capacity in Rabbit Calvarial Defects.Sci Rep. 2016 Sep 23;6:34029. doi: 10.1038/srep34029. Sci Rep. 2016. PMID: 27658481 Free PMC article.
-
Investigations into the effects of scaffold microstructure on slow-release system with bioactive factors for bone repair.Front Bioeng Biotechnol. 2023 Sep 14;11:1230682. doi: 10.3389/fbioe.2023.1230682. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37781533 Free PMC article. Review.
References
-
- U.S. Census Bureau. Interim State Population Projections. 2005.
-
- U.S. Census Bureau. Health and nutrition. Washington, DC: U.S. Census Bureau statistical abstracts of the United States; 2009. p. 117.
-
- Thawani JP, Wang AC, Than KD, Lin CY, La Marca F, Park P. Bone morphogenetic proteins and cancer: review of the literature. Neurosurgery. 2010;66:233–246. - PubMed
-
- Pasche B. Role of transforming growth factor beta in cancer. J Cell Physiol. 2001;186:153–168. - PubMed
-
- Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–1728.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

