CD4 T follicular helper cell dynamics during SIV infection
- PMID: 22922258
- PMCID: PMC3428091
- DOI: 10.1172/JCI63039
CD4 T follicular helper cell dynamics during SIV infection
Abstract
CD4 T follicular helper (TFH) cells interact with and stimulate the generation of antigen-specific B cells. TFH cell interaction with B cells correlates with production of SIV-specific immunoglobulins. However, the fate of TFH cells and their participation in SIV-induced antibody production is not well understood. We investigated the phenotype, function, location, and molecular signature of TFH cells in rhesus macaques. Similar to their human counterparts, TFH cells in rhesus macaques represented a heterogeneous population with respect to cytokine function. In a highly differentiated subpopulation of TFH cells, characterized by CD150lo expression, production of Th1 cytokines was compromised while IL-4 production was augmented, and cells exhibited decreased survival, cycling, and trafficking capacity. TFH cells exhibited a distinct gene profile that was markedly altered by SIV infection. TFH cells were infected by SIV; yet, in some animals, these cells actually accumulated during chronic SIV infection. Generalized immune activation and increased IL-6 production helped drive TFH differentiation during SIV infection. Accumulation of TFH cells was associated with increased frequency of activated germinal center B cells and SIV-specific antibodies. Therefore, chronic SIV does not disturb the ability of TFH cells to help B cell maturation and production of SIV-specific immunoglobulins.
Figures
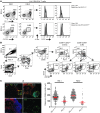
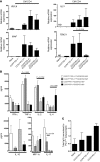
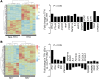

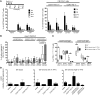

Comment in
-
HIV and T follicular helper cells: a dangerous relationship.J Clin Invest. 2012 Sep;122(9):3059-62. doi: 10.1172/JCI65175. Epub 2012 Aug 27. J Clin Invest. 2012. PMID: 22922252 Free PMC article.
References
-
- Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nat Immunol. 2011;12(6):472–477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

