Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression
- PMID: 22922260
- PMCID: PMC3428093
- DOI: 10.1172/JCI63401
Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression
Abstract
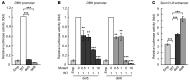
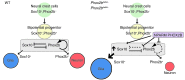
The most common forms of neurocristopathy in the autonomic nervous system are Hirschsprung disease (HSCR), resulting in congenital loss of enteric ganglia, and neuroblastoma (NB), childhood tumors originating from the sympathetic ganglia and adrenal medulla. The risk for these diseases dramatically increases in patients with congenital central hypoventilation syndrome (CCHS) harboring a nonpolyalanine repeat expansion mutation of the Paired-like homeobox 2b (PHOX2B) gene, but the molecular mechanism of pathogenesis remains unknown. We found that introducing nonpolyalanine repeat expansion mutation of the PHOX2B into the mouse Phox2b locus recapitulates the clinical features of the CCHS associated with HSCR and NB. In mutant embryos, enteric and sympathetic ganglion progenitors showed sustained sex-determining region Y (SRY) box10 (Sox10) expression, with impaired proliferation and biased differentiation toward the glial lineage. Nonpolyalanine repeat expansion mutation of PHOX2B reduced transactivation of wild-type PHOX2B on its known target, dopamine β-hydroxylase (DBH), in a dominant-negative fashion. Moreover, the introduced mutation converted the transcriptional effect of PHOX2B on a Sox10 enhancer from repression to transactivation. Collectively, these data reveal that nonpolyalanine repeat expansion mutation of PHOX2B is both a dominant-negative and gain-of-function mutation. Our results also demonstrate that Sox10 regulation by PHOX2B is pivotal for the development and pathogenesis of the autonomic ganglia.
Figures

Comment in
-
NPARM in PHOX2B: why some things just should not be expanded.J Clin Invest. 2012 Sep;122(9):3056-8. doi: 10.1172/JCI63884. Epub 2012 Aug 27. J Clin Invest. 2012. PMID: 22922261 Free PMC article.
Similar articles
-
Congenital central hypoventilation syndrome: PHOX2B mutations and phenotype.Am J Respir Crit Care Med. 2006 Nov 15;174(10):1139-44. doi: 10.1164/rccm.200602-305OC. Epub 2006 Aug 3. Am J Respir Crit Care Med. 2006. PMID: 16888290
-
Transcriptional dysregulation and impairment of PHOX2B auto-regulatory mechanism induced by polyalanine expansion mutations associated with congenital central hypoventilation syndrome.Neurobiol Dis. 2013 Feb;50:187-200. doi: 10.1016/j.nbd.2012.10.019. Epub 2012 Oct 25. Neurobiol Dis. 2013. PMID: 23103552
-
Variable phenotypes in congenital central hypoventilation syndrome with PHOX2B nonpolyalanine repeat mutations.J Clin Sleep Med. 2021 Oct 1;17(10):2049-2055. doi: 10.5664/jcsm.9370. J Clin Sleep Med. 2021. PMID: 33983112 Free PMC article.
-
Causative and common PHOX2B variants define a broad phenotypic spectrum.Clin Genet. 2020 Jan;97(1):103-113. doi: 10.1111/cge.13633. Epub 2019 Aug 30. Clin Genet. 2020. PMID: 31444792 Review.
-
PHOX2B: a diagnostic cornerstone in neurocristopathies and neuroblastomas.J Clin Pathol. 2024 May 17;77(6):378-382. doi: 10.1136/jcp-2023-209047. J Clin Pathol. 2024. PMID: 38458747 Review.
Cited by
-
A Focus on Regulatory Networks Linking MicroRNAs, Transcription Factors and Target Genes in Neuroblastoma.Cancers (Basel). 2021 Nov 3;13(21):5528. doi: 10.3390/cancers13215528. Cancers (Basel). 2021. PMID: 34771690 Free PMC article. Review.
-
Distinct neuroblastoma-associated alterations of PHOX2B impair sympathetic neuronal differentiation in zebrafish models.PLoS Genet. 2013 Jun;9(6):e1003533. doi: 10.1371/journal.pgen.1003533. Epub 2013 Jun 6. PLoS Genet. 2013. PMID: 23754957 Free PMC article.
-
Dysregulation of locus coeruleus development in congenital central hypoventilation syndrome.Acta Neuropathol. 2015 Aug;130(2):171-83. doi: 10.1007/s00401-015-1441-0. Epub 2015 May 15. Acta Neuropathol. 2015. PMID: 25975378 Free PMC article.
-
Knockdown of PHOX2B in the retrotrapezoid nucleus reduces the central CO2 chemoreflex in rats.Elife. 2024 May 10;13:RP94653. doi: 10.7554/eLife.94653. Elife. 2024. PMID: 38727716 Free PMC article.
-
Mef2c-F10N enhancer driven β-galactosidase (LacZ) and Cre recombinase mice facilitate analyses of gene function and lineage fate in neural crest cells.Dev Biol. 2015 Jun 1;402(1):3-16. doi: 10.1016/j.ydbio.2015.02.022. Epub 2015 Mar 17. Dev Biol. 2015. PMID: 25794678 Free PMC article.
References
-
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge, United Kingdom: Cambridge University Press; 1999.
-
- Etchevers HC, Amiel J, Lyonnet S. Neural Crest Induction and Differentiation. 2006. Molecular bases of human neurocristopathies. In: Saint-Jeannet JP, ed. pp. 213–234. Georgetown, Texas, USA: Landes Bioscience; - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

