Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium
- PMID: 22922364
- PMCID: PMC4005791
- DOI: 10.1038/ni.2397
Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium
Abstract
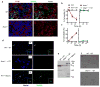
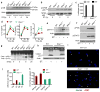
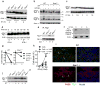
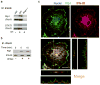
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a virulent pathogen that induces rapid host death. Here we observed that host survival after infection with S. Typhimurium was enhanced in the absence of type I interferon signaling, with improved survival of mice deficient in the receptor for type I interferons (Ifnar1(-/-) mice) that was attributed to macrophages. Although there was no impairment in cytokine expression or inflammasome activation in Ifnar1(-/-) macrophages, they were highly resistant to S. Typhimurium-induced cell death. Specific inhibition of the kinase RIP1 or knockdown of the gene encoding the kinase RIP3 prevented the death of wild-type macrophages, which indicated that necroptosis was a mechanism of cell death. Finally, RIP3-deficient macrophages, which cannot undergo necroptosis, had similarly less death and enhanced control of S. Typhimurium in vivo. Thus, we propose that S. Typhimurium induces the production of type I interferon, which drives necroptosis of macrophages and allows them to evade the immune response.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
RIPK3-Dependent Recruitment of Low-Inflammatory Myeloid Cells Does Not Protect from Systemic Salmonella Infection.mBio. 2020 Oct 6;11(5):e02588-20. doi: 10.1128/mBio.02588-20. mBio. 2020. PMID: 33024046 Free PMC article.
-
Type I interferon enhances necroptosis of Salmonella Typhimurium-infected macrophages by impairing antioxidative stress responses.J Cell Biol. 2017 Dec 4;216(12):4107-4121. doi: 10.1083/jcb.201701107. Epub 2017 Oct 20. J Cell Biol. 2017. PMID: 29055012 Free PMC article.
-
Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages.mBio. 2016 Feb 16;7(1):e02051-15. doi: 10.1128/mBio.02051-15. mBio. 2016. PMID: 26884434 Free PMC article.
-
Critical role of type I interferon-induced macrophage necroptosis during infection with Salmonella enterica serovar Typhimurium.Cell Mol Immunol. 2013 Mar;10(2):99-100. doi: 10.1038/cmi.2012.68. Epub 2012 Dec 24. Cell Mol Immunol. 2013. PMID: 23262973 Free PMC article. Review. No abstract available.
-
Type 1 interferon-associated necroptosis: a novel mechanism for Salmonella enterica Typhimurium to induce macrophage death.Cell Mol Immunol. 2013 Jan;10(1):10-2. doi: 10.1038/cmi.2012.54. Epub 2012 Nov 12. Cell Mol Immunol. 2013. PMID: 23147719 Free PMC article. Review. No abstract available.
Cited by
-
Roles of RIPK3 in necroptosis, cell signaling, and disease.Exp Mol Med. 2022 Oct;54(10):1695-1704. doi: 10.1038/s12276-022-00868-z. Epub 2022 Oct 12. Exp Mol Med. 2022. PMID: 36224345 Free PMC article. Review.
-
CYLD Proteolysis Protects Macrophages from TNF-Mediated Auto-necroptosis Induced by LPS and Licensed by Type I IFN.Cell Rep. 2016 Jun 14;15(11):2449-61. doi: 10.1016/j.celrep.2016.05.032. Epub 2016 Jun 2. Cell Rep. 2016. PMID: 27264187 Free PMC article.
-
Execution of RIPK3-regulated necrosis.Mol Cell Oncol. 2014 Oct 29;1(2):e960759. doi: 10.4161/23723548.2014.960759. eCollection 2014 Apr-Jun. Mol Cell Oncol. 2014. PMID: 27308332 Free PMC article. Review.
-
RNA Editing in Glioma as a Sexually Dimorphic Prognostic Factor That Affects mRNA Abundance in Fatty Acid Metabolism and Inflammation Pathways.Cells. 2022 Apr 5;11(7):1231. doi: 10.3390/cells11071231. Cells. 2022. PMID: 35406793 Free PMC article.
-
A non-classical monocyte-derived macrophage subset provides a splenic replication niche for intracellular Salmonella.Immunity. 2021 Dec 14;54(12):2712-2723.e6. doi: 10.1016/j.immuni.2021.10.015. Epub 2021 Nov 16. Immunity. 2021. PMID: 34788598 Free PMC article.
References
-
- Jones BD, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

