Decoding the perception of pain from fMRI using multivariate pattern analysis
- PMID: 22922369
- PMCID: PMC3532598
- DOI: 10.1016/j.neuroimage.2012.08.035
Decoding the perception of pain from fMRI using multivariate pattern analysis
Abstract
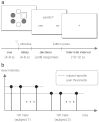
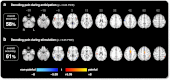
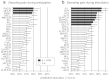
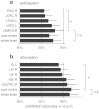
Pain is known to comprise sensory, cognitive, and affective aspects. Despite numerous previous fMRI studies, however, it remains open which spatial distribution of activity is sufficient to encode whether a stimulus is perceived as painful or not. In this study, we analyzed fMRI data from a perceptual decision-making task in which participants were exposed to near-threshold laser pulses. Using multivariate analyses on different spatial scales, we investigated the predictive capacity of fMRI data for decoding whether a stimulus had been perceived as painful. Our analysis yielded a rank order of brain regions: during pain anticipation, activity in the periaqueductal gray (PAG) and orbitofrontal cortex (OFC) afforded the most accurate trial-by-trial discrimination between painful and non-painful experiences; whereas during the actual stimulation, primary and secondary somatosensory cortex, anterior insula, dorsolateral and ventrolateral prefrontal cortex, and OFC were most discriminative. The most accurate prediction of pain perception from the stimulation period, however, was enabled by the combined activity in pain regions commonly referred to as the 'pain matrix'. Our results demonstrate that the neural representation of (near-threshold) pain is spatially distributed and can be best described at an intermediate spatial scale. In addition to its utility in establishing structure-function mappings, our approach affords trial-by-trial predictions and thus represents a step towards the goal of establishing an objective neuronal marker of pain perception.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Apkarian A.V., Bushnell M.C., Treede R.-D., Zubieta J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9(4):463–484. - PubMed
-
- Brooks J.C.W., Zambreanu L., Godinez A., Craig A.D.B., Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27(1):201–209. - PubMed
-
- Davatzikos C., Ruparel K., Fan Y., Shen D.G., Acharyya M., Loughead J.W., Gur R.C. Classifying spatial patterns of brain activity with machine learning methods: application to lie detection. Neuroimage. 2005;28(3):663–668. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

