Soluble ST2 protein inhibits LPS stimulation on monocyte-derived dendritic cells
- PMID: 22922442
- PMCID: PMC4002335
- DOI: 10.1038/cmi.2012.29
Soluble ST2 protein inhibits LPS stimulation on monocyte-derived dendritic cells
Abstract
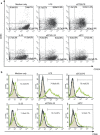
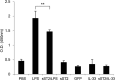
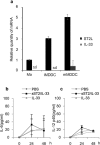
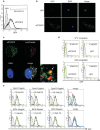
ST2 protein is a soluble splicing variant of ST2L protein, which is the receptor for interleukin-33 (IL-33). Previously, we reported that soluble ST2 suppressed the signal transduction of lipopolysaccharide (LPS) and cytokine production in monocytic cells. To investigate whether or not this inhibitory effect occurs in dendritic cells, which are the key players in innate and adaptive immunity, human monocyte-derived dendritic cells were pre-treated with soluble ST2 protein before LPS stimulation. Although soluble ST2 did not attenuate the LPS-induced maturation of dendritic cells, pre-treatment with soluble ST2 suppressed cytokine production and inhibited LPS signaling. Moreover, the proliferation of naive T cells was inhibited significantly by soluble ST2 pre-treatment. IL-33 had little effect on the cytokine production of immature monocyte-derived dendritic cells. Furthermore, soluble ST2 protein was internalized into dendritic cells, suggesting that soluble ST2 protein acts by a noncanonical mechanism other than the sequestration of IL-33.
Figures




Similar articles
-
Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation.J Biol Chem. 2007 Sep 7;282(36):26369-80. doi: 10.1074/jbc.M704916200. Epub 2007 Jul 10. J Biol Chem. 2007. PMID: 17623648
-
IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells.J Leukoc Biol. 2006 Jun;79(6):1279-85. doi: 10.1189/jlb.0905503. Epub 2006 Mar 21. J Leukoc Biol. 2006. PMID: 16551679
-
A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression.J Immunol. 2001 Jun 1;166(11):6633-9. doi: 10.4049/jimmunol.166.11.6633. J Immunol. 2001. PMID: 11359817
-
The IL-33/ST2 pathway--A new therapeutic target in cardiovascular disease.Pharmacol Ther. 2011 Aug;131(2):179-86. doi: 10.1016/j.pharmthera.2011.02.005. Epub 2011 Feb 26. Pharmacol Ther. 2011. PMID: 21356240 Review.
-
IL-33/ST2 axis in inflammation and immunopathology.Immunol Res. 2012 Apr;52(1-2):89-99. doi: 10.1007/s12026-012-8283-9. Immunol Res. 2012. PMID: 22392053 Review.
Cited by
-
Soluble St2 Induces Cardiac Fibroblast Activation and Collagen Synthesis via Neuropilin-1.Cells. 2020 Jul 10;9(7):1667. doi: 10.3390/cells9071667. Cells. 2020. PMID: 32664340 Free PMC article.
-
Soluble form of the ST2 gene product exhibits growth promoting activity in NIH-3T3 cells.Biochem Biophys Rep. 2015 Nov 21;5:8-15. doi: 10.1016/j.bbrep.2015.11.020. eCollection 2016 Mar. Biochem Biophys Rep. 2015. PMID: 28955802 Free PMC article.
-
The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases.Front Immunol. 2017 Apr 24;8:475. doi: 10.3389/fimmu.2017.00475. eCollection 2017. Front Immunol. 2017. PMID: 28484466 Free PMC article. Review.
-
A Novel Bifunctional Fusion Protein (Anti-IL-17A-sST2) Protects against Acute Liver Failure, Modulating the TLR4/MyD88 Pathway and NLRP3 Inflammasome Activation.Biomedicines. 2024 May 17;12(5):1118. doi: 10.3390/biomedicines12051118. Biomedicines. 2024. PMID: 38791080 Free PMC article.
-
Endotoxin-Binding Peptides Derived from Casein Glycomacropeptide Inhibit Lipopolysaccharide-Stimulated Inflammatory Responses via Blockade of NF-κB activation in macrophages.Nutrients. 2015 Apr 27;7(5):3119-37. doi: 10.3390/nu7053119. Nutrients. 2015. PMID: 25923657 Free PMC article.
References
-
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
-
- Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–26380. - PubMed
-
- Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–281. - PubMed
-
- Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S. Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commu. 2001;284:1104–1108. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

