Single-protein nanomechanical mass spectrometry in real time
- PMID: 22922541
- PMCID: PMC3435450
- DOI: 10.1038/nnano.2012.119
Single-protein nanomechanical mass spectrometry in real time
Abstract
Nanoelectromechanical systems (NEMS) resonators can detect mass with exceptional sensitivity. Previously, mass spectra from several hundred adsorption events were assembled in NEMS-based mass spectrometry using statistical analysis. Here, we report the first realization of single-molecule NEMS-based mass spectrometry in real time. As each molecule in the sample adsorbs on the resonator, its mass and position of adsorption are determined by continuously tracking two driven vibrational modes of the device. We demonstrate the potential of multimode NEMS-based mass spectrometry by analysing IgM antibody complexes in real time. NEMS-based mass spectrometry is a unique and promising new form of mass spectrometry: it can resolve neutral species, provide a resolving power that increases markedly for very large masses, and allow the acquisition of spectra, molecule-by-molecule, in real time.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

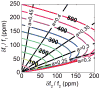
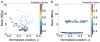


References
-
- Andersson CO. Mass Spectrometric Studies on Amino Acid and Peptide Derivatives. Acta Chem Scand. 1958;12:1353.
-
- Beynon JH. The use of the mass spectrometer for the identification of organic compounds. Microchim Acta. 1956;44:437.
-
- Domon B, Aebersold R. Mass spectrometry and protein analysis. Science. 2006;312:212–217. - PubMed
-
- Benesch JLP, Robinson CV. Mass spectrometry of macromolecular assemblies: preservation and dissociation. Curr Opin Struc Biol. 2006;16:245–251. - PubMed
-
- Robinson CV, Benesch JLP, Ruotolo BT, Simmons DA. Protein complexes in the gas phase: Technology for structural genomics and proteomics. Chem Rev. 2007;107:3544–3567. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

