The BBSome controls IFT assembly and turnaround in cilia
- PMID: 22922713
- PMCID: PMC3434251
- DOI: 10.1038/ncb2560
The BBSome controls IFT assembly and turnaround in cilia
Abstract
The bidirectional movement of intraflagellar transport (IFT) particles, which are composed of motors, IFT-A and IFT-B subcomplexes, and cargoes, is required for the biogenesis and signalling of cilia(1,2). A successful IFT cycle depends on the proper assembly of the massive IFT particle at the ciliary base and its turnaround from anterograde to retrograde transport at the ciliary tip. However, how IFT assembly and turnaround are regulated in vivo remains elusive. From a whole-genome mutagenesis screen in Caenorhabditis elegans, we identified two hypomorphic mutations in dyf-2 and bbs-1 as the only mutants showing normal anterograde IFT transport but defective IFT turnaround at the ciliary tip. Further analyses revealed that the BBSome (refs 3, 4), a group of conserved proteins affected in human Bardet-Biedl syndrome(5) (BBS), assembles IFT complexes at the ciliary base, then binds to the anterograde IFT particle in a DYF-2- (an orthologue of human WDR19) and BBS-1-dependent manner, and lastly reaches the ciliary tip to regulate proper IFT recycling. Our results identify the BBSome as the key player regulating IFT assembly and turnaround in cilia.
Figures

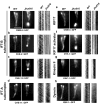
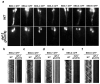
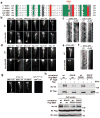

Comment in
-
Regulating intraflagellar transport.Nat Cell Biol. 2012 Sep;14(9):904-6. doi: 10.1038/ncb2569. Nat Cell Biol. 2012. PMID: 22945257
References
-
- Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Current topics in developmental biology. 2008;85:23–61. - PubMed
-
- Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. - PubMed
-
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. - PubMed
-
- Loktev AV, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Developmental cell. 2008;15:854–865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

