Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors
- PMID: 22922765
- PMCID: PMC3601133
- DOI: 10.4161/hv.21872
Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors
Abstract
Staphylococcus aureus is a major cause of healthcare-associated infections and is responsible for a substantial burden of disease in hospitalized patients. Despite increasingly rigorous infection control guidelines, the prevalence and corresponding negative impact of S. aureus infections remain considerable. Difficulties in controlling S. aureus infections as well as the associated treatment costs are exacerbated by increasing rates of resistance to available antibiotics. Despite ongoing efforts over the past 20 years, no licensed S. aureus vaccine is currently available. However, learning from past clinical failures of vaccine candidates and a better understanding of the immunopathology of S. aureus colonization and infection have aided in the design of new vaccine candidates based on multiple important bacterial pathogenesis mechanisms. This review outlines important considerations in designing a vaccine for the prevention of S. aureus disease in healthcare settings.
Keywords: Staphylococcus aureusvaccine; clinical trial; immune evasion; vaccine development; virulence factor.
Figures
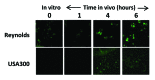
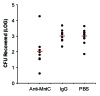

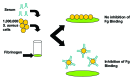
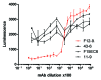
References
-
- Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med. 2005;165:1756–61. doi: 10.1001/archinte.165.15.1756. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
