Symptoms in children with convergence insufficiency: before and after treatment
- PMID: 22922781
- PMCID: PMC3461822
- DOI: 10.1097/OPX.0b013e318269c8f9
Symptoms in children with convergence insufficiency: before and after treatment
Abstract
Purpose: To investigate symptom patterns and evaluate the relationship between patient characteristics and symptom severity before and after treatment for symptomatic children with convergence insufficiency (CI).
Methods: In a randomized clinical trial, the convergence insufficiency symptom survey was administered pre- and posttreatment to 221 children aged 9 to <18 years with symptomatic CI. Frequency of symptom type was determined at baseline, mean change in performance-related vs. eye-related symptoms for treatment responders was compared, and the relationship between patient characteristics and symptom severity at baseline for the entire cohort and after treatment for those who responded to treatment was determined.
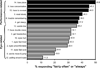
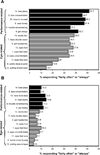
Results: At baseline, the score for performance-related symptoms was greater than that for eye-related symptoms (mean response of 2.3 vs. 1.8, p < 0.001) regardless of age, sex, race/ethnicity, or presence of parent-reported Attention Deficit Hyperactivity Disorder (ADHD). Symptom severity increased with age for both the overall and eye-related subscale scores (p = 0.048, p = 0.022, respectively). Children with parent-reported ADHD were more symptomatic (p = 0.005) than those without parent-reported ADHD because of a higher performance-related score (p < 0.001). A significant and equal improvement (p < 0.01) for the performance- and eye-related symptoms was found in treatment responders. Girls had significantly lower performance-related symptoms than boys (p = 0.014), and black children reported less eye-related symptoms than white children (p = 0.022). Children without parent-reported ADHD had significantly less symptoms overall and less eye-related symptoms than children with parent-reported ADHD (p = 0.019, p = 0.011, respectively).
Conclusions: Because of a high frequency of both performance- and eye-related symptoms, clinicians should perform a targeted history that addresses both types of symptoms to help identify children with symptomatic CI. Future study regarding the relationship of CI and symptoms and their potential influence on ADHD, reading performance, and attention is warranted.
Trial registration: ClinicalTrials.gov NCT00338611.
Figures

References
-
- Letourneau JE, Lapierre N, Lamont A. The relationship between convergence insufficiency and school achievement. Am J Optom Physiol Opt. 1979;56:18–22. - PubMed
-
- Letourneau J, Ducic S. Prevalence of convergence insufficiency among elementary school children. Can J Optom. 1988;50:194–197.
-
- Porcar E, Martinez-Palomera A. Prevalence of general binocular dysfunctions in a population of university students. Optom Vis Sci. 1997;74:111–113. - PubMed
-
- Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN, the Convergence Insufficiency and Reading Study (CIRS) group. Rouse MW, Borsting E, Hyman L, Hussein M, Cotter SA, Flynn M, Scheiman M, Gallaway M, De Land PN. Frequency of convergence insufficiency among fifth and sixth graders. Optom Vis Sci. 1999;76:643–649. - PubMed
-
- Daum KM. Convergence insufficiency. Am J Optom Physiol Opt. 1984;61:16–22. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- EY014710/EY/NEI NIH HHS/United States
- U10 EY014709/EY/NEI NIH HHS/United States
- EY014659/EY/NEI NIH HHS/United States
- U10 EY014715/EY/NEI NIH HHS/United States
- EY014709/EY/NEI NIH HHS/United States
- U10 EY014710/EY/NEI NIH HHS/United States
- EY014713/EY/NEI NIH HHS/United States
- U10 EY014659/EY/NEI NIH HHS/United States
- EY014715/EY/NEI NIH HHS/United States
- U10 EY014712/EY/NEI NIH HHS/United States
- EY014712/EY/NEI NIH HHS/United States
- U10 EY014676/EY/NEI NIH HHS/United States
- U10 EY014706/EY/NEI NIH HHS/United States
- U10 EY014713/EY/NEI NIH HHS/United States
- EY014676/EY/NEI NIH HHS/United States
- EY014706/EY/NEI NIH HHS/United States
- EY014716/EY/NEI NIH HHS/United States
- U10 EY014716/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

