STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma
- PMID: 22922995
- PMCID: PMC3583669
- DOI: 10.3892/ijo.2012.1594
STAT3 signal transduction through interleukin-22 in oral squamous cell carcinoma
Abstract
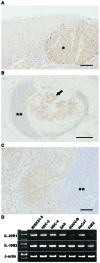
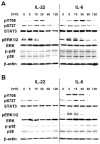
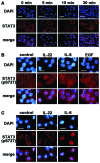
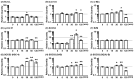
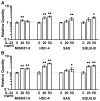
Interleukin (IL)-22 is a member of the IL-10 family. Its main targets are epithelial cells, not immune cells. We examined IL-22 signal transduction in oral squamous cell carcinoma (OSCC) cells. Immunohistochemical staining revealed that IL-22R was expressed more highly in OSCC compared to normal regions. An IL-22R signal was also observed in metastatic OSCC cells in the lymph node. RT-PCR showed that the human OSCC cell lines MISK81-5, HSC-3, HSC-4, SAS and SQUU-B expressed IL-22 receptor chains. Immunoblotting showed that IL-22 induced a transient tyrosine phosphorylation of STAT3 (pY705-STAT3) in MISK81-5 cells. The change in the serine phosphorylation of STAT3 was subtle during the examination periods. Simultaneously, pY705-STAT3 activation in HSC-3 cells was undetectable after IL-22 stimulation. Immunocytochemistry demonstrated that IL-22 induced the translocation of phosphorylated STAT3 into the nucleus of MISK81-5 cells. IL-22 temporarily upregulated the expression of anti-apoptotic and mitogenic genes such as Bcl-x, survivin and c-Myc, as well as SOCS3. IL-22 transiently activated ERK1/2 and induced a delayed phosphorylation of p38 MAP kinase, but negligibly involved the activation of NF-κB in MISK81-5 cells. MISK81-5 and SQUU-B cells treated with IL-22 showed mild cellular proliferation. MISK81-5, HSC-4 and SAS cells treated with IL-22 downregulated the keratinocyte differentiation-related genes compared with unstimulated cells. Conversely, STAT3 suppression by STAT3 siRNA strongly disrupted the downregulation of these genes by IL-22, but it did not significantly affect the activation of ERK1/2 by IL-22. The OSCC cells used in this study upregulated the expression of SERPINB3/4 (SCCA1/2), well-known SCC markers, following treatment with IL-22. These results indicate that IL-22 differentially activates the STAT3 signaling system depending on the type of OSCC. IL-22 may therefore play a role in tumor growth, cell differentiation and progression through STAT3-dependent and -independent pathways.
Figures

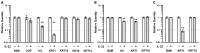

Similar articles
-
Role of tumour-associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge.Diagn Pathol. 2017 Apr 5;12(1):32. doi: 10.1186/s13000-017-0623-6. Diagn Pathol. 2017. PMID: 28381274 Free PMC article. Review.
-
Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression.PLoS One. 2013 Nov 20;8(11):e80998. doi: 10.1371/journal.pone.0080998. eCollection 2013. PLoS One. 2013. PMID: 24278362 Free PMC article.
-
Lipopolysaccharide-squamous cell carcinoma-monocyte interactions induce cancer-supporting factors leading to rapid STAT3 activation.Head Neck Pathol. 2008 Mar;2(1):1-12. doi: 10.1007/s12105-007-0038-x. Head Neck Pathol. 2008. PMID: 19603082 Free PMC article.
-
Interleukin-22 promotes the migration and invasion of oral squamous cell carcinoma cells.Immunol Med. 2020 Sep;43(3):121-129. doi: 10.1080/25785826.2020.1775060. Epub 2020 Jun 16. Immunol Med. 2020. PMID: 32546118
-
STAT3 and Its Targeting Inhibitors in Oral Squamous Cell Carcinoma.Cells. 2022 Oct 5;11(19):3131. doi: 10.3390/cells11193131. Cells. 2022. PMID: 36231093 Free PMC article. Review.
Cited by
-
Role of tumour-associated macrophages in oral squamous cells carcinoma progression: an update on current knowledge.Diagn Pathol. 2017 Apr 5;12(1):32. doi: 10.1186/s13000-017-0623-6. Diagn Pathol. 2017. PMID: 28381274 Free PMC article. Review.
-
Th22 cell accumulation is associated with colorectal cancer development.World J Gastroenterol. 2015 Apr 14;21(14):4216-24. doi: 10.3748/wjg.v21.i14.4216. World J Gastroenterol. 2015. PMID: 25892871 Free PMC article.
-
The multifaceted role of STAT3 pathway and its implication as a potential therapeutic target in oral cancer.Arch Pharm Res. 2022 Aug;45(8):507-534. doi: 10.1007/s12272-022-01398-y. Epub 2022 Aug 20. Arch Pharm Res. 2022. PMID: 35987863 Review.
-
p38 Expression and Modulation of STAT3 Signaling in Oral Cancer.Pathol Oncol Res. 2020 Jan;26(1):183-192. doi: 10.1007/s12253-018-0405-9. Epub 2018 Mar 21. Pathol Oncol Res. 2020. PMID: 29564744
-
Molecular Pathogenesis of Psoriasis and Biomarkers Reflecting Disease Activity.J Clin Med. 2021 Jul 21;10(15):3199. doi: 10.3390/jcm10153199. J Clin Med. 2021. PMID: 34361983 Free PMC article. Review.
References
-
- Chidzonga MM, Mahomva L. Squamous cell carcinoma of the oral cavity, maxillary antrum and lip in a Zimbabwean population: a descriptive epidemiological study. Oral Oncol. 2006;42:184–189. - PubMed
-
- Chidzonga MM. Oral malignant neoplasia: a survey of 428 cases in two Zimbabwean hospitals. Oral Oncol. 2006;42:177–183. - PubMed
-
- Dimery IW, Hong WK. Overview of combined modality therapies for head and neck cancer. J Natl Cancer Inst. 1993;85:95–111. - PubMed
-
- Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973–1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128:268–274. - PubMed
-
- Llewellyn CD, Linklater K, Bell J, Johnson NW, Warnakulasuriya KA. Squamous cell carcinoma of the oral cavity in patients aged 45 years and under: a descriptive analysis of 116 cases diagnosed in the South East of England from 1990 to 1997. Oral Oncol. 2003;39:106–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

