Extracardiac neural remodeling in humans with cardiomyopathy
- PMID: 22923270
- PMCID: PMC3529182
- DOI: 10.1161/CIRCEP.112.972836
Extracardiac neural remodeling in humans with cardiomyopathy
Abstract
Background: Intramyocardial nerve sprouting after myocardial infarction is associated with ventricular arrhythmias. Whether human stellate ganglia remodel in association with cardiac pathology is unknown. The purpose of this study was to determine whether cardiac pathology is associated with remodeling of the stellate ganglia in humans.
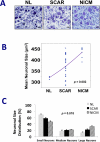
Methods and results: Left stellate ganglia were collected from patients undergoing sympathetic denervation for intractable ventricular arrhythmias and from cadavers, along with intact hearts. Clinical data on patients and cadaveric subjects were reviewed. We classified ganglia from normal, scarred, and nonischemic cardiomyopathic hearts without scar as NL (n=3), SCAR (n=24), and NICM (n=7), respectively. Within left stellate ganglia, neuronal size, density, fibrosis, synaptic density, and nerve sprouting were determined. Nerve density and sprouting were also quantified in cadaveric hearts. Mean neuronal size in normal, scarred, and nonischemic cardiomyopathic hearts without scar groups were 320 ± 4 μm(2), 372 ± 10 μm(2), and 435 ± 10 μm(2) (P=0.002), respectively. No significant differences in neuronal density and fibrosis were present between the groups. Synaptic density in ganglia from SCAR and NICM groups were 57.8 ± 11.2 μm(2)/mm(2) (P=0.084) and 44.5 ± 7.9 μm(2)/mm(2) (P=0.039), respectively, compared with the normal group, 17.8 ± 7 μm(2)/mm(2) (overall P=0.162). There were no significant differences in left stellate ganglia nerve sprouting or myocardial nerve density between the groups.
Conclusions: Neuronal hypertrophy within left stellate ganglia is associated with chronic cardiomyopathy in humans. Ganglionic and myocardial nerve sprouting and nerve density were not significantly different. These changes may be related to increased cardiac sympathetic signaling and ventricular arrhythmias. Further studies are needed to determine the electrophysiological consequences of extracardiac neuronal remodeling in humans.
Figures



References
-
- Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res. 1966;18:416–428. - PubMed
-
- Zipes DP, Barber MJ, Takahashi N, Gilmour RF. Influence of the autonomic nervous system on the genesis of cardiac arrhythmias. Pacing Clin Electrophysiol. 1983;5:1210–1220. - PubMed
-
- Opthof T, Misier A, Coronel R, Vermeulen J, Verberne H, Frank R, Moulijn A, Capelle Fv, Janse M. Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ Res. 1991;68:1204–15. - PubMed
-
- Ramirez RJ, Ajijola OA, Zhou W, Holmstrom B, Luning H, Laks MM, Shivkumar K, Mahajan A. A new electrocardiographic marker for sympathetic nerve stimulation: modulation of repolarization by stimulation of stellate ganglia. J Electrocardiol. 2011;44:694–699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

