Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools
- PMID: 22923371
- PMCID: PMC3473077
- DOI: 10.1007/s11745-012-3709-7
Dietary trans-fatty acid induced NASH is normalized following loss of trans-fatty acids from hepatic lipid pools
Abstract
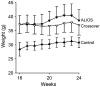
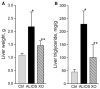
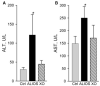
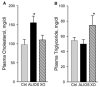
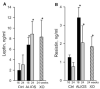
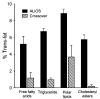
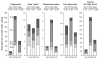
Previous experiments in mice showed that dietary trans-fats could play a role in non-alcoholic steatohepatitis (NASH) yet little is known about the accumulation trans-fats in hepatic lipid pools in relationship to liver injury. NASH is also associated with obesity yet improves with only modest weight loss. To distinguish the role of obesity versus sustained consumption of a trans-fat containing diet in causing NASH, mice with obesity and NASH induced by consuming a high trans-fat diet for 16 weeks were subsequently fed standard chow or maintained on trans-fat chow for another 8 weeks. The accumulation, partitioning and loss of trans-fats in the major hepatic lipid pools during and after trans-fat consumption were determined. Obese mice switched to standard chow remained obese but steatohepatitis improved. trans-fats were differentially incorporated into the major hepatic lipid pools and the loss of trans-fats after crossover to control chow was greatest in the cholesteryl ester pool. In summary, dietary changes can improve the biochemical and histopathological changes of NASH despite persistent obesity in mice. Analysis of hepatic lipids confirmed that dietary trans-fats accumulate in the major lipid pools and are released differentially with diet normalization. The substantial loss of trans-fats from the cholesteryl ester pool in parallel with improvement in NASH suggests that this pool of trans-fats could play a role in the pathogenesis of NASH.
Figures

References
-
- Bugianesi E, Marzocchi R, Villanova N, Marchesini G. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): treatment. Baillieres Best Pract Res Clin Gastroenterol. 2004;18:1105–1116. - PubMed
-
- Neuschwander-Tetri BA. Lifestyle modification as the primary treatment of NASH. Clin Liver Dis. 2009;13:649–665. - PubMed
-
- Haynes P, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis. 2004;8:535–547. - PubMed
-
- Silverman JF, O’Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, Norris HT, Caro JF. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. - PubMed
-
- Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S, Torimura T, Inuzuka S, Sata M, Tanikawa K. Therapeutic effects of restricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27:103–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

