The endosomal protein-sorting receptor sortilin has a role in trafficking α-1 antitrypsin
- PMID: 22923381
- PMCID: PMC3522165
- DOI: 10.1534/genetics.112.143487
The endosomal protein-sorting receptor sortilin has a role in trafficking α-1 antitrypsin
Abstract
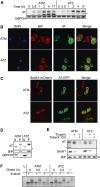
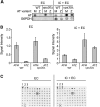
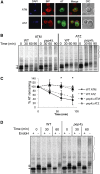
Up to 1 in 3000 individuals in the United States have α-1 antitrypsin deficiency, and the most common cause of this disease is homozygosity for the antitrypsin-Z variant (ATZ). ATZ is inefficiently secreted, resulting in protein deficiency in the lungs and toxic polymer accumulation in the liver. However, only a subset of patients suffer from liver disease, suggesting that genetic factors predispose individuals to liver disease. To identify candidate factors, we developed a yeast ATZ expression system that recapitulates key features of the disease-causing protein. We then adapted this system to screen the yeast deletion mutant collection to identify conserved genes that affect ATZ secretion and thus may modify the risk for developing liver disease. The results of the screen and associated assays indicate that ATZ is degraded in the vacuole after being routed from the Golgi. In fact, one of the strongest hits from our screen was Vps10, which can serve as a receptor for the delivery of aberrant proteins to the vacuole. Because genome-wide association studies implicate the human Vps10 homolog, sortilin, in cardiovascular disease, and because hepatic cell lines that stably express wild-type or mutant sortilin were recently established, we examined whether ATZ levels and secretion are affected by sortilin. As hypothesized, sortilin function impacts the levels of secreted ATZ in mammalian cells. This study represents the first genome-wide screen for factors that modulate ATZ secretion and has led to the identification of a gene that may modify disease severity or presentation in individuals with ATZ-associated liver disease.
Figures


References
-
- Adams A., Gottschling D. E., Kaiser C. A., Stearns T., 1997. Media and stock preservation, pp. 145–160 in Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Amberg D. C., Burke D. J., Strathern J. N., 2005. Yeast immunofluorescence, pp. 149–152 in Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Bathurst I. C., Travis J., George P. M., Carrell R. W., 1984. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 177: 179–183. - PubMed
-
- Bathurst I. C., Errington D. M., Foreman R. C., Judah J. D., Carrell R. W., 1985. Human Z alpha 1-antitrypsin accumulates intracellularly and stimulates lysosomal activity when synthesised in the Xenopus oocyte. FEBS Lett. 183: 304–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

