Enzymatic glycosylation of nonbenzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase
- PMID: 22923401
- PMCID: PMC3485733
- DOI: 10.1128/AEM.02004-12
Enzymatic glycosylation of nonbenzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase
Abstract
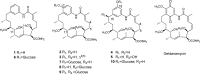
Geldanamycin (GM) is a naturally occurring anticancer agent isolated from several strains of Streptomyces hygroscopicus. However, its potential clinical utility is compromised by its severe toxicity and poor water solubility. For this reason, considerable efforts are under way to make new derivatives that have both good clinical efficacy and high water solubility. On the other hand, glycosylation is often a step that improves the water solubility and/or biological activity in many natural products of biosynthesis. Here, we report the facile production of glucose-conjugated nonbenzoquinone GM analogs using the Bacillus UDP-glycosyltransferase BL-C. Five aglycon substrates containing nonbenzoquinone aromatic rings were chosen to validate the in vitro glycosylation reaction. Putative glucoside compounds were determined through the presence of a product peak(s) and were also verified using LC/MS analyses. Further, the chemical structures of new glucoside compounds 6 and 7 were elucidated using spectroscopy data. These glucoside compounds showed a dramatic improvement in water solubility compared with that of the original aglycon, nonbenzoquinone GM.
Figures




Similar articles
-
Biosynthesis of Novel Glucosides Geldanamycin Analogs by Enzymatic Synthesis.J Microbiol Biotechnol. 2016 Jan;26(1):56-60. doi: 10.4014/jmb.1508.08069. J Microbiol Biotechnol. 2016. PMID: 26464380
-
New geldanamycin analogs from Streptomyces hygroscopicus.J Microbiol Biotechnol. 2012 Nov;22(11):1478-81. doi: 10.4014/jmb.1206.06026. J Microbiol Biotechnol. 2012. PMID: 23124337
-
Enzymatic biosynthesis and biological evaluation of novel 17-AAG glucoside as potential anti-cancer agents.Bioorg Med Chem Lett. 2020 Aug 1;30(15):127282. doi: 10.1016/j.bmcl.2020.127282. Epub 2020 May 21. Bioorg Med Chem Lett. 2020. PMID: 32527461
-
Enzymatic biosynthesis of novel neobavaisoflavone glucosides via Bacillus UDP-glycosyltransferase.Chin J Nat Med. 2017 Apr;15(4):281-287. doi: 10.1016/S1875-5364(17)30045-6. Chin J Nat Med. 2017. PMID: 28527513
-
Leloir glycosyltransferases of natural product C-glycosylation: structure, mechanism and specificity.Biochem Soc Trans. 2020 Aug 28;48(4):1583-1598. doi: 10.1042/BST20191140. Biochem Soc Trans. 2020. PMID: 32657344 Review.
Cited by
-
Herbimycins D-F, ansamycin analogues from Streptomyces sp. RM-7-15.J Nat Prod. 2013 Sep 27;76(9):1619-26. doi: 10.1021/np400308w. Epub 2013 Aug 15. J Nat Prod. 2013. PMID: 23947794 Free PMC article.
-
Enzymatic Biosynthesis of Novel Resveratrol Glucoside and Glycoside Derivatives.Appl Environ Microbiol. 2014 Dec;80(23):7235-43. doi: 10.1128/AEM.02076-14. Epub 2014 Sep 19. Appl Environ Microbiol. 2014. PMID: 25239890 Free PMC article.
-
Purification and characterization of a novel phloretin-2'-O-glycosyltransferase favoring phloridzin biosynthesis.Sci Rep. 2016 Oct 12;6:35274. doi: 10.1038/srep35274. Sci Rep. 2016. PMID: 27731384 Free PMC article.
-
OleD Loki as a Catalyst for Hydroxamate Glycosylation.Chembiochem. 2020 Apr 1;21(7):952-957. doi: 10.1002/cbic.201900601. Epub 2019 Nov 26. Chembiochem. 2020. PMID: 31621997 Free PMC article.
-
Phylogeny-guided characterization of glycosyltransferases for epothilone glycosylation.Microb Biotechnol. 2019 Jul;12(4):763-774. doi: 10.1111/1751-7915.13421. Epub 2019 May 8. Microb Biotechnol. 2019. PMID: 31069998 Free PMC article.
References
-
- Ahn BC, et al. 2009. Formation of flavone di-O-glucosides using a glycosyltransferase from Bacillus cereus. J. Microbiol. Biotech. 19:387–390 - PubMed
-
- Biamonte MA, et al. 2010. Heat shock protein 90: inhibitors in clinical trials. J. Med. Chem. 53:3–17 - PubMed
-
- Cheng H, et al. 2005. Synthesis and enzyme-specific activation of carbohydrate-geldanamycin conjugates with potent anticancer activity. J. Med. Chem. 48:645–652 - PubMed
-
- Cysyk RL, et al. 2006. Reaction of geldanamycin and C17-substituted analogues with glutathione: product identifications and pharmacological implications. Chem. Res. Toxicol. 19:376–381 - PubMed
-
- DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. 1970. Geldanamycin, a new antibiotic. J. Antibiot. (Tokyo) 23:442–447 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

