Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal Guinea pig organs
- PMID: 22923417
- PMCID: PMC3676261
- DOI: 10.1177/1933719112453509
Chronic hypoxia impairs cytochrome oxidase activity via oxidative stress in selected fetal Guinea pig organs
Abstract
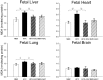
We hypothesized that chronic hypoxia disrupts mitochondrial function via oxidative stress in fetal organs. Pregnant guinea pig sows were exposed to either normoxia or hypoxia (10.5% O2, 14 days) in the presence or absence of the antioxidant, N-acetylcysteine (NAC). Near-term anesthetized fetuses were delivered via hysterotomy, and fetal livers, hearts, lungs, and forebrains harvested. We quantified the effects of chronic hypoxia on cytochrome oxidase (CCO) activity and 2 factors known to regulate CCO activity: malondialdehyde (MDA) and CCO subunit 4 (COX4). Hypoxia increased the MDA levels in fetal liver, heart, and lung with a corresponding reduction in CCO activity, prevented by prenatal NAC. The COX4 expression paralleled CCO activity in fetal liver and lung, but was unaltered in fetal hearts due to hypoxia. Hypoxia reduced the brain COX4 expression despite having no effect on CCO activity. This study identifies the mitochondrion as an important target site in tissue-specific oxidative stress for the induction of fetal hypoxic injury.
Conflict of interest statement
Figures



References
-
- Kingdom JC, Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18(8):613–621. - PubMed
-
- Gilbert WM, Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol. 2003;188(6):1596–1599; discussion 1599-1601. - PubMed
-
- Kramer MS, Séguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinat Epidemiol. 2000;14(3):194–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

