Pathogenesis of age-related bone loss in humans
- PMID: 22923429
- PMCID: PMC3826857
- DOI: 10.1093/gerona/gls163
Pathogenesis of age-related bone loss in humans
Abstract
Background: Although data from rodent systems are extremely useful in providing insights into possible mechanisms of age-related bone loss, concepts evolving from animal models need to ultimately be tested in humans.
Methods: This review provides an update on mechanisms of age-related bone loss in humans based on the author's knowledge of the field and focused literature reviews.
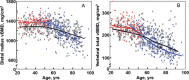
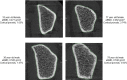
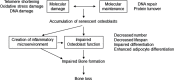
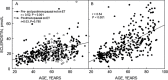
Results: Novel imaging, experimental models, biomarkers, and analytic techniques applied directly to human studies are providing new insights into the patterns of bone mass acquisition and loss as well as the role of sex steroids, in particular estrogen, on bone metabolism and bone loss with aging in women and men. These studies have identified the onset of trabecular bone loss at multiple sites that begins in young adulthood and remains unexplained, at least based on current paradigms of the mechanisms of bone loss. In addition, estrogen appears to be a major regulator of bone metabolism not only in women but also in men. Studies assessing mechanisms of estrogen action on bone in humans have identified effects of estrogen on RANKL expression by several different cell types in the bone microenvironment, a role for TNF-α and IL-1β in mediating effects of estrogen deficiency on bone, and possible regulation of the Wnt inhibitor, sclerostin, by estrogen.
Conclusions: There have been considerable advances in our understanding of age-related bone loss in humans. However, there are also significant gaps in knowledge, particularly in defining cell autonomous changes in bone in human studies to test or validate concepts emerging from studies in rodents. Decision Editor: Luigi Ferrucci, MD, PhD.
Keywords: Aging.; Bone; Estrogen; Osteoporosis.
Figures

References
-
- Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJP. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study Osteoporos Int 2001;12:623–629 - PubMed
-
- Landin LA. Fracture patterns in children. Analysis of 8,682 fractures with special reference to incidence, etiology and secular changes in a Swedish urban population 1950–1979 Acta Orthop Scand Suppl 1983;202:1–109 - PubMed
-
- Kramhoft M, Bodtker S. Epidemiology of distal forearm fractures in Danish children Acta Orthop Scand. 1988;59:557–559 - PubMed
-
- Bailey DA, Wedge JH, McCulloch RG, Martin AD, Bernhardson SC. Epidemiology of fractures of the distal end of the radius in children as associated with growth J Bone Joint Surg Am. 1989;71:1225–1231 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

