A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia
- PMID: 22923574
- PMCID: PMC3633213
- DOI: 10.1126/science.1223012
A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia
Abstract
Mucus clearance is the primary defense mechanism that protects airways from inhaled infectious and toxic agents. In the current gel-on-liquid mucus clearance model, a mucus gel is propelled on top of a "watery" periciliary layer surrounding the cilia. However, this model fails to explain the formation of a distinct mucus layer in health or why mucus clearance fails in disease. We propose a gel-on-brush model in which the periciliary layer is occupied by membrane-spanning mucins and mucopolysaccharides densely tethered to the airway surface. This brush prevents mucus penetration into the periciliary space and causes mucus to form a distinct layer. The relative osmotic moduli of the mucus and periciliary brush layers explain both the stability of mucus clearance in health and its failure in airway disease.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

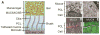

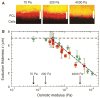
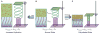
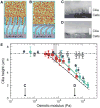
Comment in
-
Biochemistry. Walking on solid ground.Science. 2012 Aug 24;337(6097):924-5. doi: 10.1126/science.1227091. Science. 2012. PMID: 22923570 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01HL077546/HL/NHLBI NIH HHS/United States
- R01 HL103940/HL/NHLBI NIH HHS/United States
- P01 HL034322/HL/NHLBI NIH HHS/United States
- P01 HL110873/HL/NHLBI NIH HHS/United States
- P50HL107168-01/HL/NHLBI NIH HHS/United States
- P50 HL107168/HL/NHLBI NIH HHS/United States
- K01DK080847/DK/NIDDK NIH HHS/United States
- K01 DK080847/DK/NIDDK NIH HHS/United States
- P01HL110873-01/HL/NHLBI NIH HHS/United States
- R01 HL077546/HL/NHLBI NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
- UL1-RR025747/RR/NCRR NIH HHS/United States
- P30DK065988/DK/NIDDK NIH HHS/United States
- HHSN268200900020/PHS HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- P01 HL108808/HL/NHLBI NIH HHS/United States
- P50HL107168/HL/NHLBI NIH HHS/United States
- P01HL108808/HL/NHLBI NIH HHS/United States
- P01HL34322/HL/NHLBI NIH HHS/United States
- R01HL103940/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

