Phosphofructokinase 1 glycosylation regulates cell growth and metabolism
- PMID: 22923583
- PMCID: PMC3534962
- DOI: 10.1126/science.1222278
Phosphofructokinase 1 glycosylation regulates cell growth and metabolism
Abstract
Cancer cells must satisfy the metabolic demands of rapid cell growth within a continually changing microenvironment. We demonstrated that the dynamic posttranslational modification of proteins by O-linked β-N-acetylglucosamine (O-GlcNAcylation) is a key metabolic regulator of glucose metabolism. O-GlcNAcylation was induced at serine 529 of phosphofructokinase 1 (PFK1) in response to hypoxia. Glycosylation inhibited PFK1 activity and redirected glucose flux through the pentose phosphate pathway, thereby conferring a selective growth advantage on cancer cells. Blocking glycosylation of PFK1 at serine 529 reduced cancer cell proliferation in vitro and impaired tumor formation in vivo. These studies reveal a previously uncharacterized mechanism for the regulation of metabolic pathways in cancer and a possible target for therapeutic intervention.
Figures
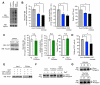
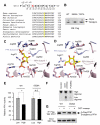
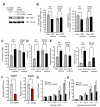

Comment in
-
Cancer. Glycosylation to adapt to stress.Science. 2012 Aug 24;337(6097):925-6. doi: 10.1126/science.1227513. Science. 2012. PMID: 22923571 No abstract available.
References
-
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
-
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85. - PubMed
-
- Christofk HR, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumor growth. Nature. 2008;452:230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

