Francisella DnaK inhibits tissue-nonspecific alkaline phosphatase
- PMID: 22923614
- PMCID: PMC3481318
- DOI: 10.1074/jbc.M112.404400
Francisella DnaK inhibits tissue-nonspecific alkaline phosphatase
Abstract
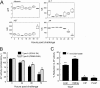
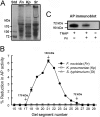
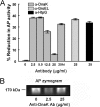
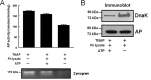
Following pulmonary infection with Francisella tularensis, we observed an unexpected but significant reduction of alkaline phosphatase, an enzyme normally up-regulated following inflammation. However, no reduction was observed in mice infected with a closely related gram-negative pneumonic organism (Klebsiella pneumoniae) suggesting the inhibition may be Francisella-specific. In similar fashion to in vivo observations, addition of Francisella lysate to exogenous alkaline phosphatase (tissue-nonspecific isozyme) was inhibitory. Partial purification and subsequent proteomic analysis indicated the inhibitory factor to be the heat shock protein DnaK. Incubation with increasing amounts of anti-DnaK antibody reduced the inhibitory effect in a dose-dependent manner. Furthermore, DnaK contains an adenosine triphosphate binding domain at its N terminus, and addition of adenosine triphosphate enhances dissociation of DnaK with its target protein, e.g. alkaline phosphatase. Addition of adenosine triphosphate resulted in decreased DnaK co-immunoprecipitated with alkaline phosphatase as well as reduction of Francisella-mediated alkaline phosphatase inhibition further supporting the binding of Francisella DnaK to alkaline phosphatase. Release of DnaK via secretion and/or bacterial cell lysis into the extracellular milieu and inhibition of plasma alkaline phosphatase could promote an orchestrated, inflammatory response advantageous to Francisella.
Figures

Similar articles
-
The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding.Biochemistry. 2001 Mar 13;40(10):3016-26. doi: 10.1021/bi002656a. Biochemistry. 2001. PMID: 11258915
-
Long-lasting recall response of CD4+ and CD8+ alphabeta T cells, but not gammadelta T cells, to heat shock proteins of francisella tularensis.Scand J Infect Dis. 2001;33(2):145-52. doi: 10.1080/003655401750065562. Scand J Infect Dis. 2001. PMID: 11233852
-
Dissociation between the critical role of ClpB of Francisella tularensis for the heat shock response and the DnaK interaction and its important role for efficient type VI secretion and bacterial virulence.PLoS Pathog. 2020 Apr 10;16(4):e1008466. doi: 10.1371/journal.ppat.1008466. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32275693 Free PMC article.
-
Role and mechanism of the Hsp70 molecular chaperone machines in bacterial pathogens.J Med Microbiol. 2017 Mar;66(3):259-265. doi: 10.1099/jmm.0.000429. Epub 2017 Mar 23. J Med Microbiol. 2017. PMID: 28086078 Review.
-
GrpE, a nucleotide exchange factor for DnaK.Cell Stress Chaperones. 2003 Fall;8(3):218-24. doi: 10.1379/1466-1268(2003)008<0218:ganeff>2.0.co;2. Cell Stress Chaperones. 2003. PMID: 14984054 Free PMC article. Review.
References
-
- Tärnvik A. (1989) Nature of protective immunity to Francisella tularensis. Rev. Infect. Dis. 11, 440–451 - PubMed
-
- Cong Y., Yu J. J., Guentzel M. N., Berton M. T., Seshu J., Klose K. E., Arulanandam B. P. (2009) Vaccination with a defined Francisella tularensis subsp. novicida pathogenicity island mutant (DeltaiglB) induces protective immunity against homotypic and heterotypic challenge. Vaccine 27, 5554–5561 - PMC - PubMed
-
- Conlan J. W., Shen H., Golovliov I., Zingmark C., Oyston P. C., Chen W., House R. V., Sjöstedt A. (2010) Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria. Effects of host background and route of immunization. Vaccine 28, 1824–1831 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

