Oxygen dose responsiveness of human fetal airway smooth muscle cells
- PMID: 22923637
- PMCID: PMC3469631
- DOI: 10.1152/ajplung.00037.2012
Oxygen dose responsiveness of human fetal airway smooth muscle cells
Abstract
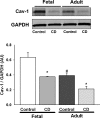
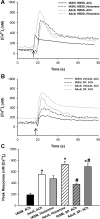
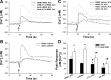
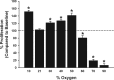
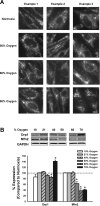
Maintenance of blood oxygen saturation dictates supplemental oxygen administration to premature infants, but hyperoxia predisposes survivors to respiratory diseases such as asthma. Although much research has focused on oxygen effects on alveoli in the setting of bronchopulmonary dysplasia, the mechanisms by which oxygen affects airway structure or function relevant to asthma are still under investigation. We used isolated human fetal airway smooth muscle (fASM) cells from 18-20 postconceptual age lungs (canalicular stage) to examine oxygen effects on intracellular Ca(2+) ([Ca(2+)](i)) and cellular proliferation. fASM cells expressed substantial smooth muscle actin and myosin and several Ca(2+) regulatory proteins but not fibroblast or epithelial markers, profiles qualitatively comparable to adult human ASM. Fluorescence Ca(2+) imaging showed robust [Ca(2+)](i) responses to 1 μM acetylcholine (ACh) and 10 μM histamine (albeit smaller and slower than adult ASM), partly sensitive to zero extracellular Ca(2+). Compared with adult, fASM showed greater baseline proliferation. Based on this validation, we assessed fASM responses to 10% hypoxia through 90% hyperoxia and found enhanced proliferation at <60% oxygen but increased apoptosis at >60%, effects accompanied by appropriate changes in proliferative vs. apoptotic markers and enhanced mitochondrial fission at >60% oxygen. [Ca(2+)](i) responses to ACh were enhanced for <60% but blunted at >60% oxygen. These results suggest that hyperoxia has dose-dependent effects on structure and function of developing ASM, which could have consequences for airway diseases of childhood. Thus detrimental effects on ASM should be an additional consideration in assessing risks of supplemental oxygen in prematurity.
Figures




References
-
- Agani FH, Kuo NT, Chang CH, Dreshaj IA, Farver CF, Krause JE, Ernsberger P, Haxhiu MA, Martin RJ. Effect of hyperoxia on substance P expression and airway reactivity in the developing lung. Am J Physiol Lung Cell Mol Physiol 273: L40–L45, 1997 - PubMed
-
- Belik J, Jankov RP, Pan J, Yi M, Chaudhry I, Tanswell AK. Chronic O2 exposure in the newborn rat results in decreased pulmonary arterial nitric oxide release and altered smooth muscle response to isoprostane. J Appl Physiol 96: 725–730, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

