ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium
- PMID: 22923691
- PMCID: PMC3465416
- DOI: 10.1073/pnas.1212834109
ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium
Abstract
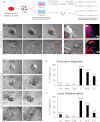
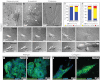
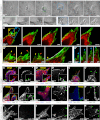
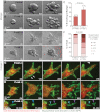
Breast cancer progression involves genetic changes and changes in the extracellular matrix (ECM). To test the importance of the ECM in tumor cell dissemination, we cultured epithelium from primary human breast carcinomas in different ECM gels. We used basement membrane gels to model the normal microenvironment and collagen I to model the stromal ECM. In basement membrane gels, malignant epithelium either was indolent or grew collectively, without protrusions. In collagen I, epithelium from the same tumor invaded with protrusions and disseminated cells. Importantly, collagen I induced a similar initial response of protrusions and dissemination in both normal and malignant mammary epithelium. However, dissemination of normal cells into collagen I was transient and ceased as laminin 111 localized to the basal surface, whereas dissemination of carcinoma cells was sustained throughout culture, and laminin 111 was not detected. Despite the large impact of ECM on migration strategy, transcriptome analysis of our 3D cultures revealed few ECM-dependent changes in RNA expression. However, we observed many differences between normal and malignant epithelium, including reduced expression of cell-adhesion genes in tumors. Therefore, we tested whether deletion of an adhesion gene could induce sustained dissemination of nontransformed cells into collagen I. We found that deletion of P-cadherin was sufficient for sustained dissemination, but exclusively into collagen I. Our data reveal that metastatic tumors preferentially disseminate in specific ECM microenvironments. Furthermore, these data suggest that breaks in the basement membrane could induce invasion and dissemination via the resulting direct contact between cancer cells and collagen I.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. - PubMed
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Friedl P, et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995;55:4557–4560. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

