A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry
- PMID: 22923785
- PMCID: PMC3623016
- DOI: 10.1177/1087057112456875
A selective ATP-binding cassette subfamily G member 2 efflux inhibitor revealed via high-throughput flow cytometry
Abstract
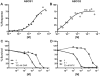
Chemotherapeutics tumor resistance is a principal reason for treatment failure, and clinical and experimental data indicate that multidrug transporters such as ATP-binding cassette (ABC) B1 and ABCG2 play a leading role by preventing cytotoxic intracellular drug concentrations. Functional efflux inhibition of existing chemotherapeutics by these pumps continues to present a promising approach for treatment. A contributing factor to the failure of existing inhibitors in clinical applications is limited understanding of specific substrate/inhibitor/pump interactions. We have identified selective efflux inhibitors by profiling multiple ABC transporters against a library of small molecules to find molecular probes to further explore such interactions. In our primary screening protocol using JC-1 as a dual-pump fluorescent reporter substrate, we identified a piperazine-substituted pyrazolo[1,5-a]pyrimidine substructure with promise for selective efflux inhibition. As a result of a focused structure-activity relationship (SAR)-driven chemistry effort, we describe compound 1 (CID44640177), an efflux inhibitor with selectivity toward ABCG2 over ABCB1. Compound 1 is also shown to potentiate the activity of mitoxantrone in vitro as well as preliminarily in vivo in an ABCG2-overexpressing tumor model. At least two analogues significantly reduce tumor size in combination with the chemotherapeutic topotecan. To our knowledge, low nanomolar chemoreversal activity coupled with direct evidence of efflux inhibition for ABCG2 is unprecedented.
Figures

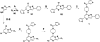

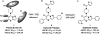
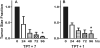
Similar articles
-
Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2.Cancer Res. 2008 Oct 1;68(19):7905-14. doi: 10.1158/0008-5472.CAN-08-0499. Cancer Res. 2008. PMID: 18829547 Free PMC article.
-
Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters.Biochem Pharmacol. 2009 Jul 15;78(2):153-61. doi: 10.1016/j.bcp.2009.04.002. Epub 2009 Apr 11. Biochem Pharmacol. 2009. PMID: 19427995
-
Effect of ceritinib (LDK378) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo.Oncotarget. 2015 Dec 29;6(42):44643-59. doi: 10.18632/oncotarget.5989. Oncotarget. 2015. PMID: 26556876 Free PMC article.
-
Selective Efflux Inhibition of ATP-binding Cassette Sub-family G Member 2.2010 Nov 30 [updated 2013 Feb 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Nov 30 [updated 2013 Feb 25]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 23658968 Free Books & Documents. Review.
-
Updated chemical scaffolds of ABCG2 inhibitors and their structure-inhibition relationships for future development.Eur J Med Chem. 2022 Nov 5;241:114628. doi: 10.1016/j.ejmech.2022.114628. Epub 2022 Jul 31. Eur J Med Chem. 2022. PMID: 35944339 Review.
Cited by
-
Novel ABCG2 Antagonists Reverse Topotecan-Mediated Chemotherapeutic Resistance in Ovarian Carcinoma Xenografts.Mol Cancer Ther. 2016 Dec;15(12):2853-2862. doi: 10.1158/1535-7163.MCT-15-0789. Epub 2016 Sep 26. Mol Cancer Ther. 2016. PMID: 27671528 Free PMC article.
-
A High-Throughput Screen of a Library of Therapeutics Identifies Cytotoxic Substrates of P-glycoprotein.Mol Pharmacol. 2019 Nov;96(5):629-640. doi: 10.1124/mol.119.115964. Epub 2019 Sep 12. Mol Pharmacol. 2019. PMID: 31515284 Free PMC article.
-
How Open Data Shapes In Silico Transporter Modeling.Molecules. 2017 Mar 7;22(3):422. doi: 10.3390/molecules22030422. Molecules. 2017. PMID: 28272367 Free PMC article. Review.
-
A high throughput flow cytometric assay platform targeting transporter inhibition.Drug Discov Today Technol. 2014 Jun;12:e95-103. doi: 10.1016/j.ddtec.2014.03.010. Drug Discov Today Technol. 2014. PMID: 25027381 Free PMC article. Review.
-
A Phenylfurocoumarin Derivative Reverses ABCG2-Mediated Multidrug Resistance In Vitro and In Vivo.Int J Mol Sci. 2021 Nov 19;22(22):12502. doi: 10.3390/ijms222212502. Int J Mol Sci. 2021. PMID: 34830383 Free PMC article.
References
-
- Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: Participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. - PubMed
-
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci. 2000;11:265–283. - PubMed
-
- Gillet JP, Efferth T, Remacle J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim Biophys Acta. 2007;1775:237–262. - PubMed
-
- Szakacs G, Varadi A, Ozvegy-Laczka C, Sarkadi B. The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME-Tox) Drug Discov Today. 2008;13:379–393. - PubMed
-
- Eckford PDW, Sharom FJ. ABC efflux pump-based resistance to chemotherapy drugs. Chem Rev. 2009;109:2989–3011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

