High-throughput FRET assay yields allosteric SERCA activators
- PMID: 22923787
- PMCID: PMC3721969
- DOI: 10.1177/1087057112456878
High-throughput FRET assay yields allosteric SERCA activators
Abstract
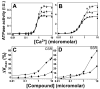
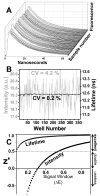
Using fluorescence resonance energy transfer (FRET), we performed a high-throughput screen (HTS) in a reconstituted membrane system, seeking compounds that reverse inhibition of sarcoplasmic reticulum Ca-ATPase (SERCA) by its cardiac regulator, phospholamban (PLB). Such compounds have long been sought to correct aberrant Ca(2+) regulation in heart failure. Donor-SERCA was reconstituted in phospholipid membranes with or without acceptor-PLB, and FRET was measured in a steady-state fluorescence microplate reader. A 20 000-compound library was tested in duplicate. Compounds that decreased FRET by more than three standard deviations were considered hits. From 43 hits (0.2%), 31 (72%) were found to be false-positives upon more thorough FRET testing. The remaining 12 hits were tested in assays of Ca-ATPase activity, and six of these activated SERCA significantly, by as much as 60%, and several also enhanced cardiomyocyte contractility. These compounds directly activated SERCA from heart and other tissues. These results validate our FRET approach and set the stage for medicinal chemistry and preclinical testing. We were concerned about the high rate of false-positives, resulting from the low precision of steady-state fluorescence. Preliminary studies with a novel fluorescence lifetime plate reader show 20-fold higher precision. This instrument can dramatically increase the quality of future HTS.
Figures


Similar articles
-
Targeting protein-protein interactions for therapeutic discovery via FRET-based high-throughput screening in living cells.Sci Rep. 2018 Aug 22;8(1):12560. doi: 10.1038/s41598-018-29685-z. Sci Rep. 2018. PMID: 30135432 Free PMC article.
-
Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer.Biochemistry. 2004 Jul 13;43(27):8754-65. doi: 10.1021/bi049732k. Biochemistry. 2004. PMID: 15236584
-
Phospholamban C-terminal residues are critical determinants of the structure and function of the calcium ATPase regulatory complex.J Biol Chem. 2014 Sep 12;289(37):25855-66. doi: 10.1074/jbc.M114.562579. Epub 2014 Jul 29. J Biol Chem. 2014. PMID: 25074938 Free PMC article.
-
SR Ca(2+)-ATPase/phospholamban in cardiomyocyte function.J Card Fail. 1996 Dec;2(4 Suppl):S77-85. doi: 10.1016/s1071-9164(96)80062-5. J Card Fail. 1996. PMID: 8951564 Review.
-
The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA).Can J Physiol Pharmacol. 2015 Oct;93(10):843-54. doi: 10.1139/cjpp-2014-0463. Epub 2015 Jan 19. Can J Physiol Pharmacol. 2015. PMID: 25730320 Review.
Cited by
-
1,4-Benzothiazepines with Cyclopropanol Groups and Their Structural Analogues Exhibit Both RyR2-Stabilizing and SERCA2a-Stimulating Activities.J Med Chem. 2023 Dec 14;66(23):15761-15775. doi: 10.1021/acs.jmedchem.3c01235. Epub 2023 Nov 22. J Med Chem. 2023. PMID: 37991191 Free PMC article.
-
Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes.Cell Metab. 2015 Sep 1;22(3):381-97. doi: 10.1016/j.cmet.2015.06.010. Epub 2015 Jul 16. Cell Metab. 2015. PMID: 26190652 Free PMC article. Review.
-
New N-aryl-N-alkyl-thiophene-2-carboxamide compound enhances intracellular Ca2+ dynamics by increasing SERCA2a Ca2+ pumping.Biophys J. 2023 Jan 17;122(2):386-396. doi: 10.1016/j.bpj.2022.12.002. Epub 2022 Dec 5. Biophys J. 2023. PMID: 36463408 Free PMC article.
-
Targeting protein-protein interactions for therapeutic discovery via FRET-based high-throughput screening in living cells.Sci Rep. 2018 Aug 22;8(1):12560. doi: 10.1038/s41598-018-29685-z. Sci Rep. 2018. PMID: 30135432 Free PMC article.
-
Stimulation of Ca2+ -ATPase Transport Activity by a Small-Molecule Drug.ChemMedChem. 2021 Nov 5;16(21):3293-3299. doi: 10.1002/cmdc.202100350. Epub 2021 Aug 26. ChemMedChem. 2021. PMID: 34297466 Free PMC article.
References
-
- Cantilina T, Sagara Y, Inesi G, Jones LR. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+ J Biol Chem. 1993;268:17018–25. - PubMed
-
- Kimura Y, Kurzydlowski K, Tada M, MacLennan DH. Phospholamban regulates the Ca2+-ATPase through intramembrane interactions. J Biol Chem. 1996;271:21726–31. - PubMed
-
- Karim CB, Marquardt CG, Stamm JD, Barany G, Thomas DD. Synthetic null-cysteine phospholamban analogue and the corresponding transmembrane domain inhibit the Ca-ATPase. Biochemistry. 2000;39:10892–7. - PubMed
-
- Lockwood NA, Tu RS, Zhang Z, Tirrell MV, Thomas DD, Karim CB. Structure and function of integral membrane protein domains resolved by peptide-amphiphiles: application to phospholamban. Biopolymers. 2003;69:283–92. - PubMed
-
- Simmerman HK, Collins JH, Theibert JL, Wegener AD, Jones LR. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986;261:13333–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

